Molecular Mesh Buildup Around Hunger Neurons Drives Obesity in Mice
The article discusses how obesity is driven by a buildup of a molecular meshwork called the extracellular matrix around neurons in the arcuate nucleus of the hypothalamus (ARC). This meshwork prevents insulin, which is secreted by the pancreas after a meal, from reaching its receptor on these neurons.
Normally, insulin signaling in the ARC helps regulate food intake, fat burning, and energy expenditure. However, when neurons lose sensitivity to insulin due to this extracellular matrix buildup, the mice start eating more, accumulating more fat, and developing metabolic issues.
The researchers found that by freeing the neurons from this molecular meshwork, they were able to restore insulin signaling and dramatically reduce the body weight of the obese mice. This suggests that targeting the extracellular matrix around ARC neurons could be a potential therapeutic approach for treating obesity and related metabolic disorders.
تخصيص الملخص
إعادة الكتابة بالذكاء الاصطناعي
إنشاء الاستشهادات
ترجمة المصدر
إلى لغة أخرى
إنشاء خريطة ذهنية
من محتوى المصدر
زيارة المصدر
www.nature.com
Obesity is driven by a build-up of molecular mesh around hunger neurons
الرؤى الأساسية المستخلصة من
by Alexander Di... في www.nature.com 09-18-2024
https://www.nature.com/articles/d41586-024-02325-5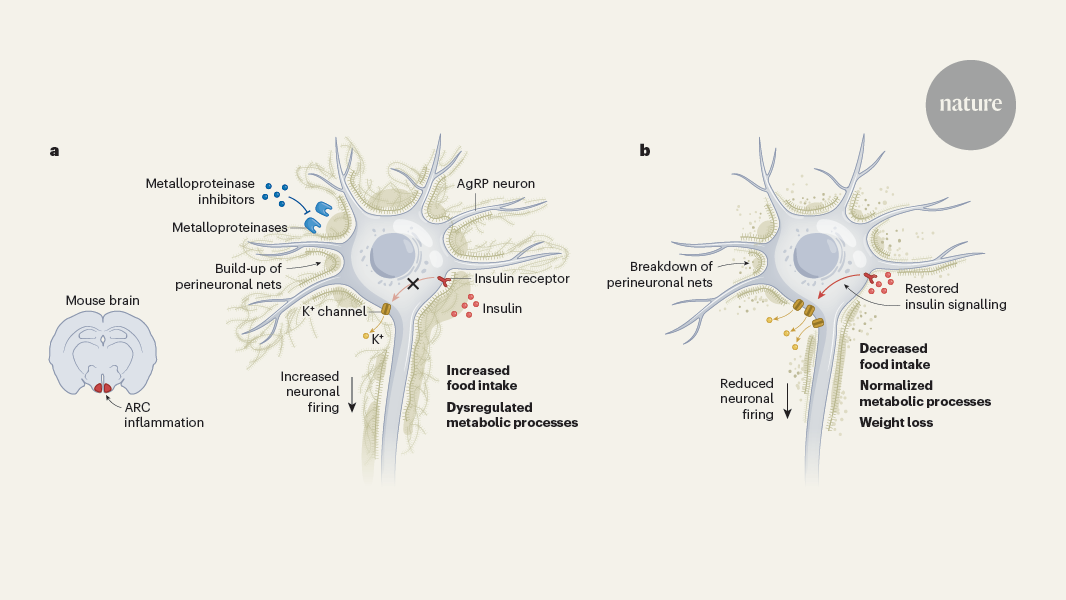
استفسارات أعمق
