Mapping Cell Surface Tensions and Contractility Mechanisms During Human Embryo Compaction
Conceptos Básicos
Increased tension at the cell-medium interface, driven by cell contractility, is the key mechanical driver of human embryo compaction, a critical early developmental process.
Resumen
The content explores the mechanical mechanisms underlying human embryo compaction, a crucial early developmental process where cells come into close contact. Using micropipette aspiration on human embryos donated for research, the study mapped cell surface tensions during compaction.
The key findings are:
- There is a fourfold increase in tension at the cell-medium interface during compaction, while cell-cell contacts maintain steady tension.
- The increased cell-medium tension, driven by cell contractility, is the primary driver of human embryo compaction.
- Comparison to mouse embryos shows human embryos use qualitatively similar but quantitatively different mechanical strategies, with human embryos being mechanically less efficient.
- Inhibition experiments demonstrate that while both cell contractility and cell-cell adhesion are required for compaction, only contractility controls the surface tensions responsible for the compaction process.
- Analysis of naturally failing human embryos indicates that defective contractility leads to non-compaction or partial compaction with excluded cells.
- The study concludes that an evolutionarily conserved increase in cell contractility is the key mechanical force driving the first morphogenetic movement that shapes the human body.
Personalizar resumen
Reescribir con IA
Generar citas
Traducir fuente
A otro idioma
Generar mapa mental
del contenido fuente
Ver fuente
www.nature.com
Mechanics of human embryo compaction - Nature
Estadísticas
There was a fourfold increase in tension at the cell-medium interface during human embryo compaction.
Cell-cell contacts maintained steady tension during human embryo compaction.
Human embryos use qualitatively similar but quantitatively different mechanical strategies compared to mouse embryos, with human embryos being mechanically less efficient.
Citas
"Increased tension at the cell–medium interface drives human embryo compaction, which is qualitatively similar to compaction in mouse embryos."
"Inhibition of cell contractility and cell–cell adhesion in human embryos shows that, whereas both cellular processes are required for compaction, only contractility controls the surface tensions responsible for compaction."
"Analysing the mechanical signature of naturally failing embryos, we find evidence that non-compacting or partially compacting embryos containing excluded cells have defective contractility."
Ideas clave extraídas de
by Juli... a las www.nature.com 05-01-2024
https://www.nature.com/articles/s41586-024-07351-x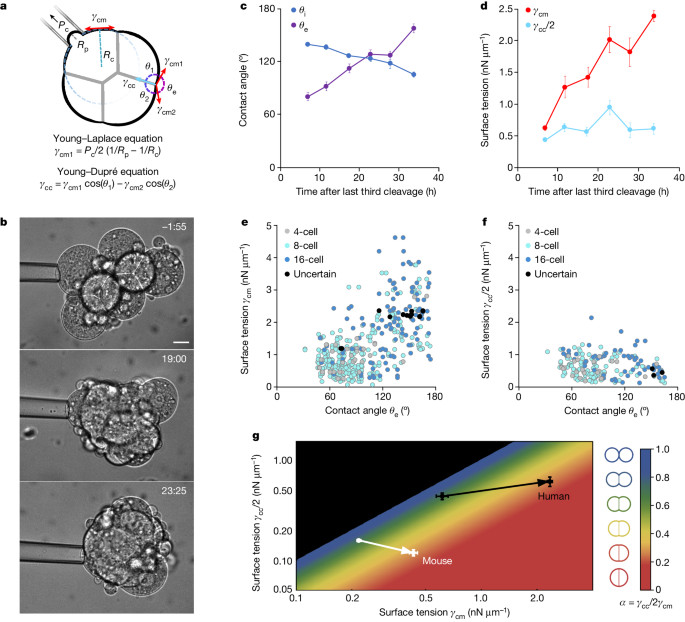
Consultas más profundas
How do the mechanical strategies for embryo compaction differ across other mammalian species, and what evolutionary factors may have shaped these differences?
In comparing the mechanical strategies for embryo compaction across mammalian species, it is evident that there are qualitative similarities but quantitative differences. For instance, the study highlights that human embryos exhibit a fourfold increase in tension at the cell-medium interface during compaction, whereas cell-cell contacts maintain a steady tension. This contrasts with mouse embryos, where compaction is qualitatively similar but more mechanically efficient. These differences could be shaped by evolutionary factors related to the reproductive strategies of each species. For example, the unique reproductive physiology and developmental timeline of humans compared to mice may have led to the evolution of slightly different mechanical strategies for embryo compaction to ensure successful development and implantation in the uterus.
What are the potential implications of the observed mechanical inefficiency in human embryo compaction compared to mouse, and how might this impact early human development and assisted reproductive technologies?
The observed mechanical inefficiency in human embryo compaction compared to mice could have significant implications for early human development and assisted reproductive technologies. The study suggests that human embryos are mechanically less efficient in compaction, which may impact the overall success rate of early development and implantation. This inefficiency could potentially lead to a higher rate of embryo failure or developmental abnormalities in humans compared to mice. In the context of assisted reproductive technologies, understanding these mechanical differences is crucial for improving the outcomes of procedures such as in vitro fertilization. By addressing the specific mechanical challenges faced by human embryos during compaction, researchers and clinicians can potentially enhance the success rates of assisted reproduction and reduce the risk of developmental issues.
Could the insights into the role of cell contractility in embryo compaction inform the development of novel interventions or treatments for infertility or early developmental disorders?
The insights gained from studying the role of cell contractility in embryo compaction have the potential to inform the development of novel interventions or treatments for infertility and early developmental disorders. The study highlights that cell contractility plays a crucial role in generating the forces necessary for embryo compaction, indicating its significance in early embryonic development. By understanding the mechanical signatures associated with faulty cell contractility, researchers may identify new targets for intervention in cases of infertility or developmental disorders. For instance, therapies that modulate cell contractility could be explored as a way to improve embryo compaction and increase the chances of successful development. Additionally, the findings could lead to the development of more targeted treatments for conditions affecting early embryogenesis, offering new possibilities for improving reproductive outcomes and addressing developmental disorders.
0
