Discovery of MicroRNAs and Their Role in Gene Regulation Earns Scientists the Nobel Prize in Medicine
This article celebrates the Nobel Prize in Medicine awarded to Victor Ambros and Gary Ruvkun for their groundbreaking discovery of microRNAs and their role in gene regulation.
The Basics of Gene Regulation
The article starts by explaining the central dogma of molecular biology: genetic information flows from DNA to RNA to protein. It emphasizes that different cell types express specific sets of proteins, highlighting the importance of precise gene regulation. This regulation ensures that only the necessary genes are active in each cell type, enabling them to perform their specialized functions.
The Breakthrough with Roundworms
Ambros and Ruvkun's research focused on the roundworm C. elegans, a model organism favored for its simple yet multicellular structure. They investigated genes controlling the timing of cell development and encountered two mutated worm strains with developmental defects. Their work revealed that a surprisingly short RNA molecule, later named microRNA, was responsible for inhibiting the activity of a specific gene.
A New Paradigm in Gene Regulation
This discovery, initially met with skepticism, unveiled a novel mechanism of gene regulation. MicroRNAs, by binding to messenger RNA (mRNA), could effectively silence or degrade the mRNA, thereby preventing protein production. This finding challenged the prevailing understanding of gene regulation, which primarily focused on transcription factors.
The Far-Reaching Impact of MicroRNAs
Further research demonstrated the widespread presence of microRNAs across various species, highlighting their evolutionary significance in shaping complex organisms. The article emphasizes that microRNAs play a crucial role in normal development and that their dysregulation can contribute to diseases like cancer, deafness, and skeletal disorders.
The Legacy of the Discovery
The discovery of microRNAs has opened up new avenues for understanding gene regulation and its implications for human health. This breakthrough has paved the way for developing potential therapeutic interventions targeting microRNAs to treat a wide range of diseases.
Personalizar resumen
Reescribir con IA
Generar citas
Traducir fuente
A otro idioma
Generar mapa mental
del contenido fuente
Ver fuente
www.medscape.com
Nobel Prize in Medicine Awarded to MicroRNA Researchers
Ideas clave extraídas de
by Michael Van ... a las www.medscape.com 10-08-2024
https://www.medscape.com/viewarticle/nobel-prize-medicine-awarded-microrna-researchers-2024a1000ido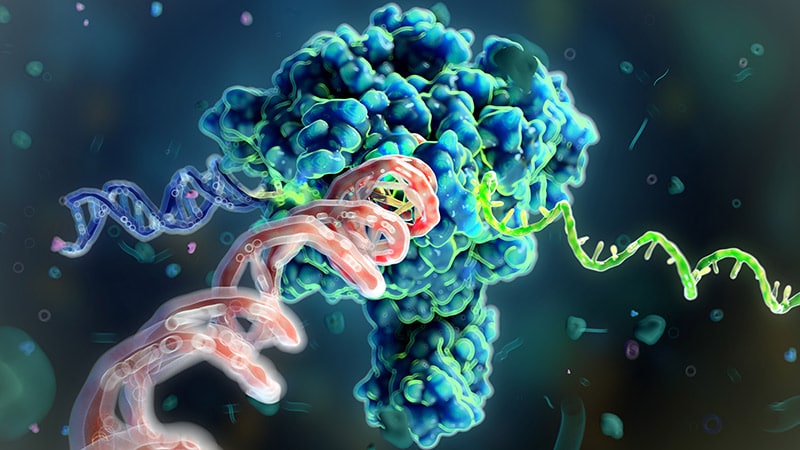
Consultas más profundas
