FLVCR1 Protein Transports Choline and Ethanolamine into Cells, Enabling Phospholipid Synthesis via the Kennedy Pathway
Alapfogalmak
FLVCR1 protein transports extracellular choline and ethanolamine into cells, enabling their phosphorylation and subsequent incorporation into phosphatidylcholine and phosphatidylethanolamine via the Kennedy pathway.
Kivonat
The content describes how the FLVCR1 protein plays a crucial role in the synthesis of the two most abundant phospholipids in mammalian cells, phosphatidylcholine and phosphatidylethanolamine.
Key highlights:
- The Kennedy pathway is the primary de novo synthesis route for these essential phospholipids, using choline and ethanolamine as precursors.
- However, the mechanisms enabling the cellular uptake of choline and ethanolamine were previously unknown.
- The study shows that the FLVCR1 protein transports extracellular choline and ethanolamine into cells, allowing their phosphorylation and subsequent incorporation into phospholipids via the Kennedy pathway.
- Structural analysis reveals that FLVCR1 binds choline and ethanolamine at a common site, but interacts with them differently due to their structural differences.
- Structure-guided mutagenesis identified residues crucial for ethanolamine transport but dispensable for choline transport, enabling functional separation of the two branches of the Kennedy pathway.
- Overall, the findings demonstrate how FLVCR1 serves as the common origin for phospholipid biosynthesis by the two branches of the Kennedy pathway.
Összefoglaló testreszabása
Átírás mesterséges intelligenciával
Hivatkozások generálása
Forrás fordítása
Egy másik nyelvre
Gondolattérkép létrehozása
a forrásanyagból
Forrás megtekintése
www.nature.com
Structural basis of lipid head group entry to the Kennedy pathway by FLVCR1 - Nature
Statisztikák
Phosphatidylcholine and phosphatidylethanolamine are the two most abundant phospholipids in mammalian cells.
The Kennedy pathway is the primary de novo synthesis route for these essential phospholipids.
Idézetek
"Structures of FLVCR1 in the presence of choline and ethanolamine reveal that both metabolites bind to a common binding site comprising aromatic and polar residues."
"Structure-guided mutagenesis identified residues that are crucial for the transport of ethanolamine, but dispensable for choline transport, enabling functional separation of the entry points into the two branches of the Kennedy pathway."
Főbb Kivonatok
by Yeeun Son,Ti... : www.nature.com 05-01-2024
https://www.nature.com/articles/s41586-024-07374-4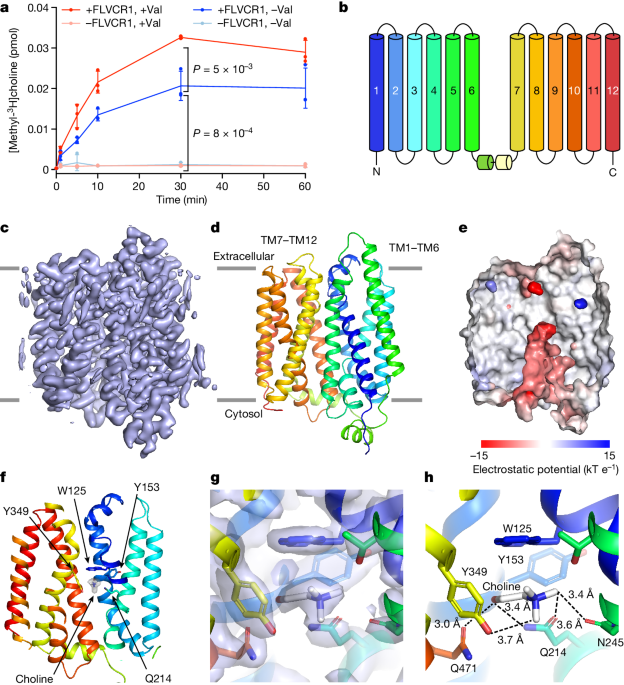
Mélyebb kérdések
What other cellular processes or pathways might be impacted by the disruption of FLVCR1-mediated choline and ethanolamine transport?
Disruption of FLVCR1-mediated choline and ethanolamine transport could potentially impact various cellular processes and pathways beyond the Kennedy pathway. Choline is not only a precursor for phosphatidylcholine synthesis but also plays a crucial role in acetylcholine production, a neurotransmitter essential for neuromuscular signaling. Therefore, a deficiency in choline uptake mediated by FLVCR1 could affect neurotransmission and lead to neurological dysfunction. Ethanolamine, on the other hand, is not only involved in phospholipid synthesis but also serves as a precursor for the synthesis of phosphatidylethanolamine, which is crucial for mitochondrial function and autophagy. Disruption of ethanolamine transport by FLVCR1 could impact mitochondrial health and cellular quality control mechanisms, potentially leading to metabolic disorders and cellular stress responses.
How might the structural differences in the FLVCR1 binding interactions with choline versus ethanolamine be exploited for the development of selective modulators of the Kennedy pathway branches?
The structural differences in FLVCR1 binding interactions with choline and ethanolamine provide a valuable opportunity for the development of selective modulators targeting the Kennedy pathway branches. By understanding the specific residues and structural features that enable FLVCR1 to distinguish between choline and ethanolamine, researchers can design small molecules or peptides that selectively interfere with either choline or ethanolamine transport while leaving the other pathway unaffected. For example, compounds that mimic the binding interactions of ethanolamine with FLVCR1 could be developed to specifically inhibit ethanolamine transport, thereby modulating the phosphatidylethanolamine branch of the Kennedy pathway without affecting phosphatidylcholine synthesis. This targeted approach could offer new therapeutic strategies for conditions where dysregulation of phospholipid metabolism is implicated.
Given the essential role of phospholipids in cellular membranes, how could a deeper understanding of the Kennedy pathway regulation contribute to our knowledge of cellular homeostasis and potential therapeutic interventions?
A deeper understanding of Kennedy pathway regulation and the role of FLVCR1 in choline and ethanolamine transport can significantly contribute to our knowledge of cellular homeostasis and open up avenues for potential therapeutic interventions. Since phospholipids are fundamental components of cellular membranes, any dysregulation in their synthesis can have profound effects on membrane integrity, cell signaling, and organelle function. By elucidating the mechanisms by which FLVCR1 mediates choline and ethanolamine uptake, researchers can uncover novel targets for therapeutic intervention in conditions where phospholipid metabolism is disrupted, such as neurodegenerative diseases, cancer, and metabolic disorders. Targeting FLVCR1 or its regulatory pathways could offer new opportunities for modulating phospholipid biosynthesis, restoring cellular membrane composition, and potentially ameliorating disease states associated with phospholipid imbalances. This deeper understanding of Kennedy pathway regulation could pave the way for the development of precision therapies that target specific aspects of phospholipid metabolism to restore cellular homeostasis and promote health.
0
