Structural Insights into the Assembly and Signaling Mechanisms of the High-Affinity IgE Receptor FcεRI Complex
Alapfogalmak
The cryogenic electron microscopy structure of the Fcε–FcεRI complex reveals the molecular basis of FcεRI assembly and the asymmetric architecture of the FcRγ dimer, which is crucial for the signaling mechanisms of FcεRI and other immune receptors.
Kivonat
The content provides structural insights into the high-affinity IgE receptor, FcεRI, which is central to the effector functions of IgE in allergic responses. The key highlights are:
- FcεRIα plays an essential role in the assembly of the tetrameric FcεRI complex, interacting with FcεRIβ and the two FcRγ subunits.
- FcεRIβ is structured as a compact four-helix bundle, similar to the B cell antigen CD20.
- The FcRγ dimer exhibits an asymmetric architecture and coils with the transmembrane region of FcεRIα to form a three-helix bundle.
- A cholesterol-like molecule enhances the interaction between FcεRIβ and the FcεRIα–FcRγ complex.
- Mutagenesis analyses reveal similarities between the interaction of FcRγ with FcεRIα and FcγRIIIA, but differences in its interaction with FcαRI.
- These findings deepen the understanding of the signaling mechanisms of FcεRI and offer insights into the functionality of other immune receptors dependent on FcRγ.
Összefoglaló testreszabása
Átírás mesterséges intelligenciával
Hivatkozások generálása
Forrás fordítása
Egy másik nyelvre
Gondolattérkép létrehozása
a forrásanyagból
Forrás megtekintése
www.nature.com
Structural insights into the high-affinity IgE receptor FcεRI complex - Nature
Statisztikák
FcεRI is a tetrameric complex, comprising FcεRIα, FcεRIβ and a homodimer of FcRγ.
FcRγ is a crucial component of other immunoglobulin receptors, including those for IgG (FcγRI and FcγRIIIA) and IgA (FcαRI).
Idézetek
"FcεRIα has an essential role in the receptor's assembly, interacting with FcεRIβ and both FcRγ subunits."
"FcεRIβ is structured as a compact four-helix bundle, similar to the B cell antigen CD20."
"The FcRγ dimer exhibits an asymmetric architecture, and coils with the transmembrane region of FcεRIα to form a three-helix bundle."
"A cholesterol-like molecule enhances the interaction between FcεRIβ and the FcεRIα–FcRγ complex."
Főbb Kivonatok
by Meijie Deng,... : www.nature.com 08-21-2024
https://www.nature.com/articles/s41586-024-07864-5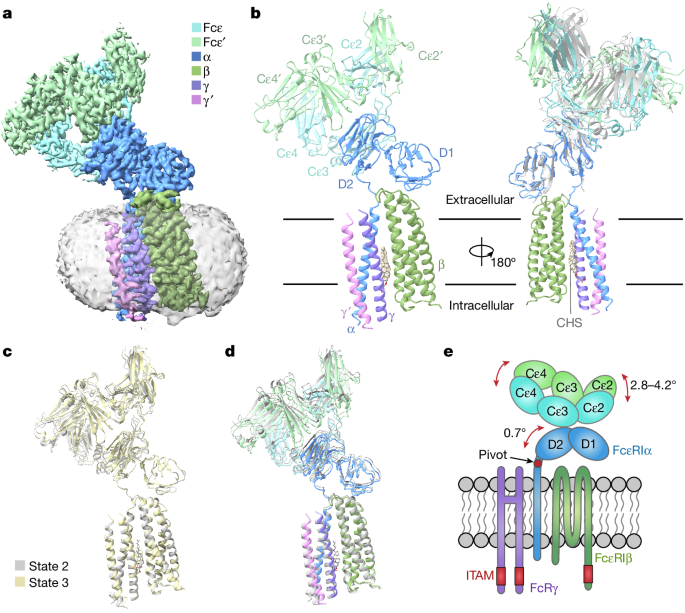
Mélyebb kérdések
How do the structural insights into the FcεRI complex inform the development of new therapeutic strategies for treating allergic diseases?
The structural insights into the FcεRI complex provide a detailed understanding of the molecular interactions involved in IgE-mediated allergic responses. By elucidating the assembly of the FcεRI complex, particularly the interactions between FcεRIα, FcεRIβ, and FcRγ, researchers can identify potential targets for therapeutic intervention. For instance, the identification of a cholesterol-like molecule that enhances the interaction between FcεRIβ and the FcεRIα–FcRγ complex could be exploited to develop molecules that disrupt this interaction, thereby inhibiting the downstream signaling cascade triggered by IgE binding to FcεRI. Additionally, the knowledge gained from the structural insights can guide the design of small molecules or biologics that selectively target specific components of the FcεRI complex, modulating its function and potentially attenuating allergic responses. Overall, these structural insights pave the way for the development of novel therapeutic strategies that target the FcεRI complex to treat allergic diseases.
What are the potential implications of the asymmetric architecture of the FcRγ dimer for the signaling mechanisms of other immune receptors that depend on FcRγ?
The asymmetric architecture of the FcRγ dimer revealed in the structural analysis of the Fcε–FcεRI complex has significant implications for the signaling mechanisms of other immune receptors that rely on FcRγ for signal transduction. The unique structural arrangement of the FcRγ dimer suggests that it may exhibit distinct modes of interaction with different receptor subunits, leading to diverse signaling outcomes. This asymmetry could allow for differential recruitment of signaling molecules or activation of specific downstream pathways depending on the receptor context. Understanding the implications of the asymmetric architecture of the FcRγ dimer is crucial for deciphering the complex signaling networks regulated by immune receptors and may provide insights into how these receptors modulate immune responses in a context-dependent manner.
Given the similarities and differences in the interaction of FcRγ with FcεRIα, FcγRIIIA, and FcαRI, how might this knowledge be leveraged to selectively modulate the activity of specific immune receptors?
The similarities and differences in the interaction of FcRγ with FcεRIα, FcγRIIIA, and FcαRI offer a valuable opportunity to selectively modulate the activity of specific immune receptors for therapeutic purposes. Leveraging this knowledge, researchers can design targeted interventions that either enhance or inhibit the interactions between FcRγ and the respective receptor subunits. For instance, if a specific immune response needs to be amplified, strategies can be developed to enhance the interaction between FcRγ and the receptor subunit, thereby promoting downstream signaling and effector functions. Conversely, if an immune response needs to be dampened, molecules can be designed to disrupt the interaction between FcRγ and the receptor subunit, thereby inhibiting signaling and attenuating the immune response. By selectively modulating the activity of specific immune receptors based on their interactions with FcRγ, tailored therapeutic approaches can be developed to address various immune-related disorders or conditions.
0
