Mechanism of BRCA1-BARD1 Complex in Regulating DNA End Resection and Protecting DNA Integrity
Concetti Chiave
The BRCA1-BARD1 complex plays a dual role in DNA repair - it directly promotes long-range DNA end resection to initiate homologous recombination, while also protecting DNA from unscheduled degradation during replication stress, with the balance between these functions determined by the presence and concentration of the recombinase RAD51.
Sintesi
The content discusses the mechanism of how the BRCA1-BARD1 complex regulates DNA repair and replication stress response. Key points:
-
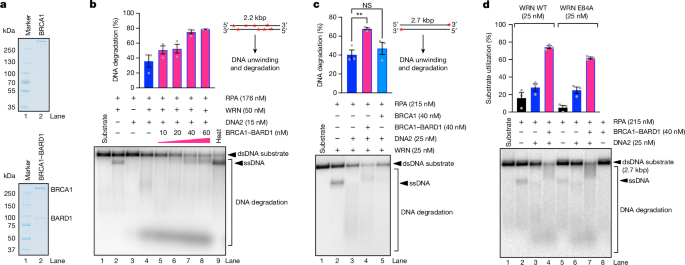
BRCA1-BARD1 directly promotes long-range DNA end resection, a critical step in initiating homologous recombination repair of DNA double-strand breaks. It stimulates the resection activity of the EXO1 and DNA2 nucleases, as well as the unwinding activity of the Werner or Bloom helicases.
-
BRCA1-BARD1 forms a complex with MRE11-RAD50-NBS1 and phosphorylated CtIP (BRCA1-C complex), which synergistically enhances the resection process.
-
Disrupting the BRCA1-C complex by mutating phosphorylated CtIP inhibits the resection function, showing the complex acts as an integrated ensemble.
-
Paradoxically, BRCA1-BARD1 also protects replication forks from unscheduled degradation during replication stress, in a homologous recombination-independent manner involving the recombinase RAD51.
-
The balance between the pro-nuclease and DNA protection functions of BRCA1-BARD1 is determined by the presence and local concentration of RAD51, depending on the physiological context.
Traduci origine
In un'altra lingua
Genera mappa mentale
dal contenuto originale
Visita l'originale
www.nature.com
Mechanism of BRCA1–BARD1 function in DNA end resection and DNA protection - Nature
Statistiche
BRCA1-BARD1 directly stimulates the resection activity of the EXO1 and DNA2 nucleases.
BRCA1-BARD1 stimulates the unwinding activity of the Werner or Bloom helicases in the DNA2-dependent resection pathway.
A mutation in phosphorylated CtIP (S327A) disrupts its binding to BRCA1 and inhibits resection, showing the BRCA1-C complex is a functionally integrated ensemble.
Citazioni
"BRCA1–BARD1 not only promotes resection and homologous recombination, but it also protects DNA upon replication stress."
"Whereas BRCA1–BARD1 stimulates resection in DSB repair, it paradoxically also protects replication forks from unscheduled degradation upon stress, which involves a homologous recombination-independent function of the recombinase RAD51."
Domande più approfondite
How do the specific protein-protein interactions within the BRCA1-C complex contribute to the synergistic enhancement of DNA end resection?
The BRCA1-C complex, which includes BRCA1, BARD1, MRE11, RAD50, NBS1, and phosphorylated CtIP, plays a crucial role in the process of DNA end resection during homologous recombination repair of DNA double-strand breaks (DSBs). The synergistic enhancement of DNA end resection is primarily facilitated through specific protein-protein interactions within this complex.
Integration of Functional Domains: The interaction between BRCA1 and phosphorylated CtIP is essential for the integrity of the BRCA1-C complex. The phosphorylation of CtIP at S327 is critical for its binding to the BRCT repeats of BRCA1. This interaction not only stabilizes the complex but also enhances the recruitment of other nucleases, such as EXO1 and DNA2, which are responsible for the degradation of the 5′-terminated strands at DSB sites.
Cooperative Action of Nucleases and Helicases: BRCA1-BARD1 directly promotes long-range DNA end resection by stimulating the activity of nucleases like EXO1 and DNA2. In the DNA2-dependent pathway, BRCA1-BARD1 enhances DNA unwinding by helicases such as Werner or Bloom helicase. This cooperative action ensures efficient resection, as helicases prepare the DNA substrate for nuclease action.
Synergistic Stimulation: The presence of MRE11-RAD50-NBS1 further amplifies the resection process. The BRCA1-C complex acts synergistically, meaning that the combined effect of these proteins is greater than the sum of their individual effects. This synergy is crucial for the rapid and effective processing of DSBs, ensuring that homologous recombination can proceed efficiently.
What are the potential mechanisms by which BRCA1-BARD1 switches between its pro-nuclease and DNA protection functions in response to the presence and concentration of RAD51?
BRCA1-BARD1 exhibits a dual functionality in DNA repair and replication stress response, which is influenced by the presence and concentration of RAD51. The potential mechanisms for this switch include:
Concentration-Dependent Binding: At low concentrations, RAD51 may promote the pro-nuclease function of BRCA1-BARD1, facilitating DNA end resection necessary for homologous recombination. However, as RAD51 concentration increases, it may bind to BRCA1-BARD1, altering its conformation and shifting its role towards DNA protection.
Competitive Inhibition: RAD51, as a recombinase, can compete with nucleases for binding to BRCA1-BARD1. When RAD51 is abundant, it may inhibit the access of nucleases to the DNA, thereby preventing degradation and promoting the stabilization of replication forks during stress conditions.
Regulatory Feedback Mechanisms: The presence of RAD51 may trigger signaling pathways that modify the activity of BRCA1-BARD1. For instance, post-translational modifications such as phosphorylation could be influenced by RAD51 binding, leading to a change in the functional state of BRCA1-BARD1 from a pro-nuclease to a protective role.
Contextual Influence: The cellular context, including the type of DNA damage and the phase of the cell cycle, may also dictate the functional outcome of BRCA1-BARD1. In scenarios where replication stress is high, the protective function may be prioritized to safeguard genomic integrity, while in the presence of DSBs, the pro-nuclease function may be favored.
Given the dual roles of BRCA1-BARD1 in DNA repair and replication stress response, how might this knowledge be leveraged for developing targeted cancer therapies?
Understanding the dual roles of BRCA1-BARD1 in DNA repair and replication stress response opens several avenues for targeted cancer therapies:
Targeting BRCA1-BARD1 Interactions: Therapeutics could be designed to modulate the interactions within the BRCA1-C complex. For instance, small molecules that enhance the binding of CtIP to BRCA1 could promote effective DNA end resection in tumors with BRCA1 mutations, potentially restoring homologous recombination repair capabilities.
Exploiting RAD51 Dynamics: Given the role of RAD51 in switching BRCA1-BARD1 functions, therapies that manipulate RAD51 levels or activity could be developed. For example, inhibiting RAD51 in BRCA1-deficient tumors may sensitize them to DNA-damaging agents by preventing the protective function of BRCA1-BARD1, thereby enhancing the efficacy of chemotherapy.
Synthetic Lethality Approaches: Leveraging the concept of synthetic lethality, drugs that target pathways compensating for BRCA1 loss (such as PARP inhibitors) could be combined with agents that disrupt the protective function of BRCA1-BARD1. This strategy could selectively kill cancer cells that rely on these pathways for survival.
Biomarker Development: The balance between the pro-nuclease and protective functions of BRCA1-BARD1 could serve as a biomarker for predicting responses to specific therapies. Understanding the expression levels of RAD51 and the status of BRCA1-BARD1 interactions could guide personalized treatment strategies.
By harnessing the intricate mechanisms of BRCA1-BARD1, targeted cancer therapies can be developed that not only improve treatment outcomes but also minimize damage to normal tissues, thereby enhancing patient quality of life.