approfondimento - Neuroscience - # Endogenous opioid regulation of ependymal cell proliferation and spinal cord scarring
Endogenous Opioid Signaling Regulates Spinal Ependymal Cell Proliferation and Scar Formation After Injury
Concetti Chiave
Endogenous κ-opioid signaling pathway involving cerebrospinal fluid-contacting neurons and prodynorphin-expressing cells regulates ependymal cell proliferation and scar formation after spinal cord injury.
Sintesi
The article explores the mechanisms underlying the regulation of ependymal cell proliferation and scar formation in the spinal cord following injury. It uncovers an endogenous κ-opioid signaling pathway that controls ependymal cell proliferation.
Key highlights:
- Ependymal cells undergo massive proliferation and differentiation into astroglia after certain spinal cord injuries, becoming a core component of the scar tissue.
- The authors detected the expression of the κ-opioid receptor OPRK1 in a cell type called cerebrospinal fluid-contacting neurons (CSF-cNs).
- They also discovered a neighboring cell population that expresses the cognate ligand prodynorphin (PDYN).
- Whereas κ-opioids are typically considered inhibitory, they excite CSF-cNs to inhibit ependymal proliferation.
- Systemic administration of a κ-antagonist enhances ependymal proliferation in uninjured spinal cords in a CSF-cN-dependent manner.
- A κ-agonist impairs ependymal proliferation, scar formation, and motor function following injury.
- The findings suggest a paracrine signaling pathway in which PDYN+ cells tonically release κ-opioids to stimulate CSF-cNs and suppress ependymal proliferation, revealing an endogenous mechanism and potential pharmacological strategy for modulating scarring after spinal cord injury.
Personalizza riepilogo
Riscrivi con l'IA
Genera citazioni
Traduci origine
In un'altra lingua
Genera mappa mentale
dal contenuto originale
Visita l'originale
www.nature.com
Endogenous opioid signalling regulates spinal ependymal cell proliferation - Nature
Statistiche
After certain spinal cord injuries, ependymal cells undergo massive proliferation and differentiation into astroglia, becoming a core component of the scar tissue.
Systemic administration of a κ-antagonist enhances ependymal proliferation in uninjured spinal cords in a CSF-cN-dependent manner.
A κ-agonist impairs ependymal proliferation, scar formation, and motor function following injury.
Citazioni
"Whereas κ-opioids are typically considered inhibitory, they excite CSF-cNs to inhibit ependymal proliferation."
"The findings suggest a paracrine signaling pathway in which PDYN+ cells tonically release κ-opioids to stimulate CSF-cNs and suppress ependymal proliferation, revealing an endogenous mechanism and potential pharmacological strategy for modulating scarring after spinal cord injury."
Approfondimenti chiave tratti da
by Wendy W. S. ... alle www.nature.com 09-18-2024
https://www.nature.com/articles/s41586-024-07889-w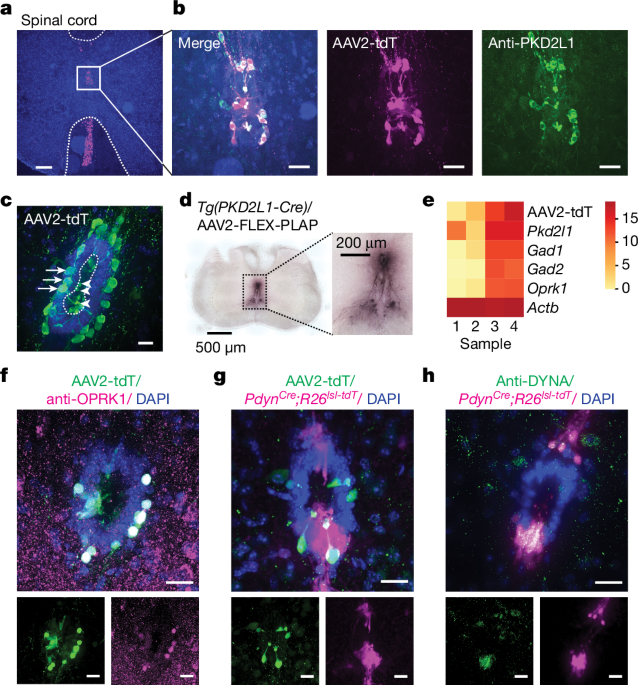
Domande più approfondite
How might the endogenous κ-opioid signaling pathway be leveraged to develop targeted therapies for spinal cord injury that optimize scar formation and neural regeneration?
The endogenous κ-opioid signaling pathway presents a promising target for developing therapies aimed at optimizing scar formation and enhancing neural regeneration following spinal cord injury. By understanding the role of κ-opioid receptors (OPRK1) and their interaction with cerebrospinal fluid-contacting neurons (CSF-cNs), researchers can design pharmacological agents that modulate this signaling pathway. For instance, the use of κ-opioid antagonists could be explored to enhance ependymal cell proliferation in uninjured spinal cords, potentially facilitating a more favorable environment for regeneration. Conversely, κ-opioid agonists could be utilized post-injury to inhibit excessive ependymal proliferation and scar formation, thereby promoting better functional recovery. This dual approach allows for a dynamic modulation of the scarring process, balancing the need for protective scar formation with the imperative of neural regeneration. Furthermore, the identification of the paracrine signaling mechanism involving prodynorphin (PDYN) and CSF-cNs opens avenues for targeted delivery systems that could selectively activate or inhibit this pathway, minimizing side effects and maximizing therapeutic efficacy.
What other cell types or signaling mechanisms might be involved in regulating ependymal cell proliferation and scar formation in the spinal cord?
In addition to the κ-opioid signaling pathway, several other cell types and signaling mechanisms are likely involved in regulating ependymal cell proliferation and scar formation in the spinal cord. Astrocytes, which have been the primary focus of scarring research, play a crucial role in the formation of the glial scar and can influence ependymal cell behavior through various cytokines and growth factors. For example, transforming growth factor-beta (TGF-β) and interleukin-6 (IL-6) are known to promote astrocyte activation and may indirectly affect ependymal cell proliferation. Additionally, microglia, the resident immune cells of the central nervous system, can release inflammatory mediators that impact both astrocytes and ependymal cells during injury. Other signaling pathways, such as the Notch and Wnt/β-catenin pathways, may also contribute to the regulation of ependymal cell fate and proliferation. Understanding the interplay between these various cell types and signaling mechanisms will be essential for developing comprehensive therapeutic strategies that address the complexities of spinal cord injury and scarring.
What are the broader implications of this study for understanding the role of cerebrospinal fluid-contacting neurons in the central nervous system and their potential therapeutic applications?
This study highlights the significant yet underexplored role of cerebrospinal fluid-contacting neurons (CSF-cNs) in the central nervous system, particularly in the context of spinal cord injury and scar formation. The discovery that CSF-cNs express κ-opioid receptors and are involved in modulating ependymal cell proliferation suggests that these neurons may serve as critical regulators of the spinal cord's response to injury. This insight opens new avenues for research into the functional roles of CSF-cNs beyond their traditional understanding as sensory neurons. Furthermore, the potential for targeting CSF-cNs in therapeutic applications could lead to innovative strategies for managing not only spinal cord injuries but also other neurological conditions characterized by scarring and inflammation. By harnessing the signaling pathways associated with CSF-cNs, researchers may develop novel interventions that promote neural repair and functional recovery, ultimately enhancing the quality of life for individuals affected by central nervous system injuries.
0
