Alanyl-tRNA Synthetases AARS1 and AARS2 Regulate cGAS Activity through Lactylation in Response to L-Lactate
핵심 개념
AARS1 and AARS2 act as intracellular l-lactate sensors and lactyltransferases that inactivate cGAS, a key regulator of innate immunity, through lactylation.
초록
The content describes how the alanyl-tRNA synthetases AARS1 and AARS2 (AARS1/2) function as intracellular sensors for l-lactate and directly catalyze the lactylation of proteins, including the cyclic GMP-AMP synthase (cGAS).
Key highlights:
- AARS1/2 and their bacterial orthologue AlaRS bind to l-lactate with micromolar affinity and catalyze ATP-dependent lactylation on lysine residues.
- In response to l-lactate, AARS2 associates with cGAS and mediates its lactylation, which inhibits cGAS liquid-like phase separation and DNA sensing abilities.
- Genetic engineering to introduce a lactyl-mimetic mutation in cGAS inhibits its function, while a lactyl-resistant mutation protects against innate immune evasion induced by high l-lactate levels.
- Blocking the lactate transporter MCT1 inhibits cGAS lactylation in stressed mice, restoring innate immune surveillance and antagonizing viral replication.
- The findings establish AARS1/2 as conserved intracellular l-lactate sensors that function as lactyltransferases, targeting and inactivating cGAS through a chemical modification process.
요약 맞춤 설정
AI로 다시 쓰기
인용 생성
소스 번역
다른 언어로
마인드맵 생성
소스 콘텐츠 기반
소스 방문
www.nature.com
AARS1 and AARS2 sense l-lactate to regulate cGAS as global lysine lactyltransferases - Nature
통계
AARS1/2 and AlaRS bind to l-lactate with micromolar affinity.
Lactylation of cGAS by AARS2 abolishes its liquid-like phase separation and DNA sensing abilities in vitro and in vivo.
Blocking the lactate transporter MCT1 inhibits cGAS lactylation in stressed mice and restores innate immune surveillance, antagonizing viral replication.
인용구
"AARS1/2 are conserved intracellular l-lactate sensors and have an essential role as lactyltransferases."
"A chemical reaction process of lactylation targets and inactivates cGAS."
핵심 통찰 요약
by Heyu Li,Chao... 게시일 www.nature.com 09-25-2024
https://www.nature.com/articles/s41586-024-07992-y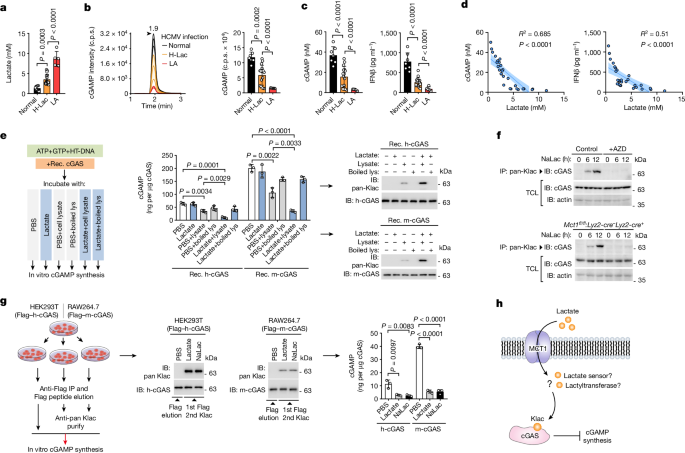
더 깊은 질문
How might the AARS1/2-mediated lactylation of other proteins, beyond cGAS, impact cellular functions and physiology?
The AARS1/2-mediated lactylation of proteins extends beyond the regulation of cGAS and could significantly influence various cellular functions and physiological processes. Lactylation, as a post-translational modification, can alter protein interactions, stability, and activity, thereby impacting metabolic pathways, gene expression, and cellular signaling. For instance, lactylation may modulate the activity of transcription factors, influencing the expression of genes involved in inflammation, metabolism, and stress responses. Additionally, the lactylation of metabolic enzymes could affect cellular energy homeostasis, particularly in conditions of hypoxia or metabolic stress where l-lactate levels are elevated. This modification could also play a role in the immune response by regulating the activity of immune-related proteins, potentially leading to altered cytokine production and immune cell activation. Overall, the AARS1/2-mediated lactylation could serve as a critical regulatory mechanism in various physiological contexts, including development, metabolism, and immune function.
What are the potential therapeutic implications of targeting the AARS1/2-cGAS lactylation axis for modulating immune responses in various disease contexts?
Targeting the AARS1/2-cGAS lactylation axis presents promising therapeutic implications for modulating immune responses in various disease contexts, particularly in autoimmune diseases, infections, and cancer. By inhibiting AARS1/2 activity or disrupting their interaction with cGAS, it may be possible to prevent the inappropriate inactivation of cGAS, thereby enhancing the innate immune response against viral infections and tumors. Conversely, in conditions characterized by excessive inflammation or autoimmunity, promoting lactylation could be a strategy to dampen cGAS activity and reduce the inflammatory response. Additionally, the use of MCT1 inhibitors to block lactate transport and subsequently reduce cGAS lactylation could restore immune surveillance in stressed tissues, offering a novel approach to cancer immunotherapy. Overall, manipulating the AARS1/2-cGAS lactylation axis could provide a versatile strategy for fine-tuning immune responses in a range of pathological conditions.
Could the AARS1/2 lactyltransferase activity be exploited for the development of novel protein engineering or post-translational modification tools?
The lactyltransferase activity of AARS1/2 holds significant potential for the development of novel protein engineering and post-translational modification tools. By harnessing the ability of AARS1/2 to catalyze the transfer of lactyl groups to specific lysine residues, researchers could create engineered proteins with tailored functionalities. This could include the design of proteins with enhanced stability, altered interaction profiles, or specific regulatory properties. Furthermore, the establishment of an orthogonal system for lactyl-lysine incorporation could facilitate the site-specific modification of proteins in living cells, allowing for precise control over protein function and activity. Such tools could be invaluable in synthetic biology, therapeutic protein design, and the study of protein dynamics in cellular contexts. Overall, the exploitation of AARS1/2 lactyltransferase activity could lead to innovative advancements in protein engineering and the understanding of post-translational modifications.
0
