inzicht - Computational Biology - # Inhibition of Mycobacterium tuberculosis and Human ATP Synthase by Bedaquiline and TBAJ-587
Structural Insights into the Inhibition of Mycobacterium tuberculosis and Human ATP Synthase by Bedaquiline and Its Analogue TBAJ-587
Belangrijkste concepten
Bedaquiline and its analogue TBAJ-587 inhibit the growth and proliferation of Mycobacterium tuberculosis by targeting the ATP synthase enzyme, but they also inhibit the human ATP synthase. This study provides structural insights into the binding mechanisms of these compounds to both the bacterial and human enzymes, which can inform the rational design of novel anti-tuberculosis drugs.
Samenvatting
The content presents a structural analysis of the interactions between the anti-tuberculosis drug bedaquiline (BDQ) and its analogue TBAJ-587 with the ATP synthase enzymes of Mycobacterium tuberculosis and humans.
Key highlights:
- BDQ and TBAJ-587 inhibit the growth and proliferation of M. tuberculosis by targeting the ATP synthase enzyme.
- However, BDQ also inhibits the human ATP synthase enzyme, which can lead to side effects.
- The study used cryogenic electron microscopy to determine the structures of M. tuberculosis ATP synthase with and without BDQ and TBAJ-587 bound, as well as the structure of human ATP synthase bound to BDQ.
- The structures reveal that BDQ and TBAJ-587 interact with specific subunits and sites within the M. tuberculosis ATP synthase, showing similar modes of action.
- The structure of human ATP synthase bound to BDQ indicates that the binding site is similar to the leading site in the M. tuberculosis enzyme, with the quinolinyl unit of BDQ making extensive contacts with the human enzyme.
- This structural information can inform the rational design of novel diarylquinoline compounds as more selective anti-tuberculosis drugs that do not inhibit the human ATP synthase.
Samenvatting aanpassen
Herschrijven met AI
Citaten genereren
Bron vertalen
Naar een andere taal
Mindmap genereren
vanuit de broninhoud
Bron bekijken
www.nature.com
Inhibition of M. tuberculosis and human ATP synthase by BDQ and TBAJ-587 - Nature
Statistieken
Bedaquiline (BDQ) is a first-in-class diarylquinoline anti-tuberculosis drug.
TBAJ-587 is an analogue of BDQ.
BDQ and TBAJ-587 prevent the growth and proliferation of Mycobacterium tuberculosis by inhibiting ATP synthase.
BDQ also inhibits human ATP synthase.
Citaten
"BDQ and TBAJ-587 have similar modes of action."
"The quinolinyl and dimethylamino units of the compounds make extensive contacts with the protein."
"The BDQ-binding site is similar to that observed for the leading site in M. tuberculosis ATP synthase, and the quinolinyl unit also interacts extensively with the human enzyme."
Belangrijkste Inzichten Gedestilleerd Uit
by Yuying Zhang... om www.nature.com 07-03-2024
https://www.nature.com/articles/s41586-024-07605-8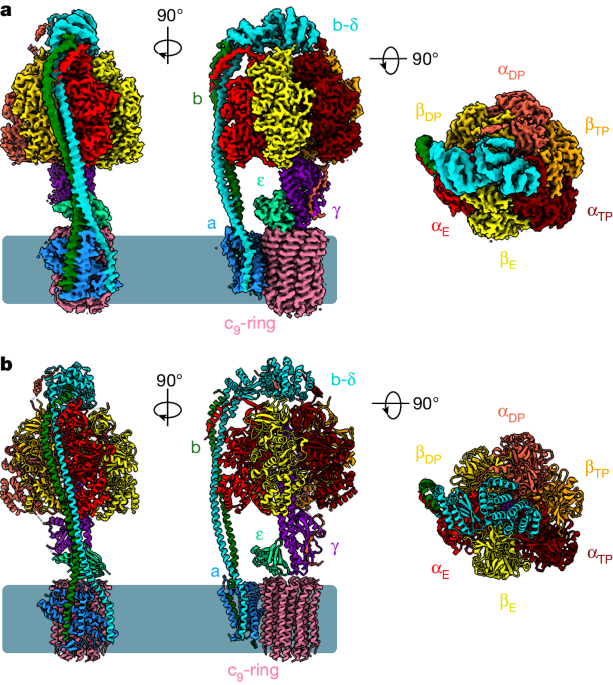
Diepere vragen
How can the structural insights from this study be leveraged to design more selective diarylquinoline compounds that do not inhibit the human ATP synthase?
The structural insights obtained from the cryogenic electron microscopy structures of M. tuberculosis ATP synthase with BDQ and TBAJ-587 bound, as well as human ATP synthase bound to BDQ, provide a detailed understanding of the binding interactions between these compounds and the enzyme subunits. By analyzing the specific regions where BDQ and TBAJ-587 interact with the ATP synthase subunits in both M. tuberculosis and human enzymes, researchers can identify key structural differences that can be exploited for designing more selective diarylquinoline compounds. By focusing on modifying the chemical structure of the compounds to target unique binding sites or alter specific functional groups that are crucial for inhibiting human ATP synthase while maintaining efficacy against M. tuberculosis ATP synthase, it is possible to develop novel compounds with improved selectivity and reduced off-target effects on human enzymes.
What other potential targets or mechanisms of action could be explored to develop anti-tuberculosis drugs that are less likely to have off-target effects on human enzymes?
To develop anti-tuberculosis drugs with reduced off-target effects on human enzymes, researchers can explore alternative targets or mechanisms of action that are specific to Mycobacterium tuberculosis and distinct from essential human cellular processes. One potential approach is to target unique metabolic pathways or enzymes that are essential for the survival and replication of M. tuberculosis but are not present or significantly different in human cells. For example, enzymes involved in the biosynthesis of cell wall components or regulators of bacterial gene expression could serve as promising targets for developing selective anti-tuberculosis drugs. By identifying and validating these novel targets through biochemical and structural studies, researchers can design inhibitors that specifically disrupt bacterial functions without affecting essential human enzymes, thereby minimizing off-target effects and improving the safety profile of anti-tuberculosis therapies.
Given the importance of ATP synthase in both bacterial and human cells, what other cellular processes or pathways could be investigated to find alternative approaches for treating tuberculosis without disrupting essential human functions?
In addition to targeting ATP synthase, researchers can explore alternative cellular processes or pathways in Mycobacterium tuberculosis that are essential for bacterial survival and virulence but are not shared or have distinct features in human cells. One potential strategy is to focus on disrupting specific signaling pathways or protein-protein interactions that are critical for bacterial pathogenesis, such as those involved in host immune evasion or antibiotic resistance mechanisms. By elucidating the molecular mechanisms underlying these pathways and identifying key regulatory proteins or enzymes that are unique to M. tuberculosis, researchers can develop targeted inhibitors or modulators that selectively interfere with bacterial functions without impacting essential processes in human cells. Furthermore, investigating the interplay between bacterial metabolism, stress responses, and virulence factors can uncover new vulnerabilities that can be exploited for developing alternative approaches to treat tuberculosis while minimizing the risk of disrupting essential human functions.
0
