Strand-Specific Patterns of DNA Damage, Repair, and Mutagenesis Reveal Insights into Cancer Genome Evolution
Grunnleggende konsepter
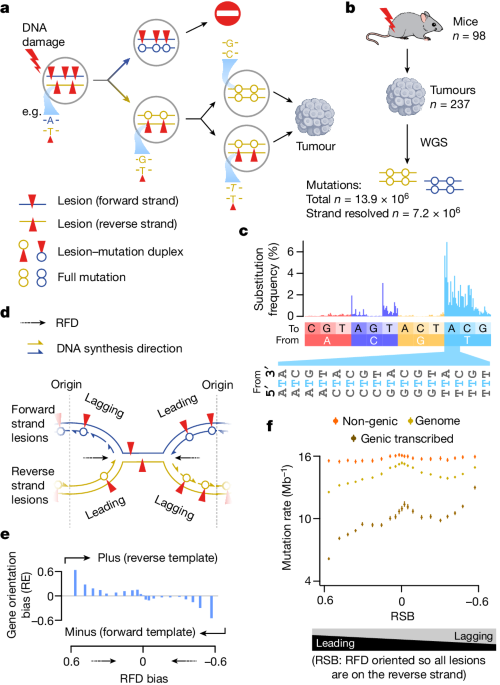
DNA damage and repair processes shape the accumulation of mutations in cancer genomes through strand-asymmetric mechanisms.
Sammendrag
The article explores how strand-asymmetric processes, such as replication and transcription, influence the patterns of DNA damage, repair, and mutagenesis. Key insights:
-
Despite distinct mechanisms of leading and lagging strand replication, the fidelity and damage tolerance are identical for both strands, suggesting the same translesion polymerase is recruited on-the-fly to both strands.
-
The accumulation of multiple distinct mutations at the site of persistent lesions provides a means to quantify the relative efficiency of repair processes genome-wide and at single-base resolution.
-
DNA damage-induced mutations are largely shaped by the influence of DNA accessibility on repair efficiency, rather than gradients of DNA damage.
-
Specific genomic conditions can actively drive oncogenic mutagenesis by corrupting the fidelity of nucleotide excision repair.
The findings provide insights into how strand-asymmetric mechanisms underlie the formation, tolerance, and repair of DNA damage, thereby shaping cancer genome evolution.
Oversett kilde
Til et annet språk
Generer tankekart
fra kildeinnhold
Besøk kilde
www.nature.com
Strand-resolved mutagenicity of DNA damage and repair - Nature
Statistikk
DNA base damage is a major source of oncogenic mutations.
Lesion segregation can produce strand-phased mutation patterns and multiallelic variation.
Leading and lagging strand replication have distinct mechanisms, but identical fidelity and damage tolerance.
The same translesion polymerase is recruited on-the-fly to both replication strands for small alkylation adducts.
Persistent lesions can accumulate multiple distinct mutations, enabling quantification of repair efficiency.
DNA damage-induced mutations are shaped by DNA accessibility, not gradients of DNA damage.
Specific genomic conditions can corrupt the fidelity of nucleotide excision repair, driving oncogenic mutagenesis.
Sitater
"Despite distinct mechanisms of leading and lagging strand replication3,4, we observe identical fidelity and damage tolerance for both strands."
"The accumulation of multiple distinct mutations at the site of persistent lesions provides the means to quantify the relative efficiency of repair processes genome wide and at single-base resolution."
"At multiple scales, we show DNA damage-induced mutations are largely shaped by the influence of DNA accessibility on repair efficiency, rather than gradients of DNA damage."
Dypere Spørsmål
How do the findings on strand-specific DNA damage and repair patterns vary across different cancer types and genomic contexts?
The findings on strand-specific DNA damage and repair patterns can vary significantly across different cancer types and genomic contexts. Different cancer types may exhibit distinct mutation signatures resulting from specific DNA damage repair mechanisms. For example, certain cancers may show a higher prevalence of mutations associated with UV-induced adducts due to exposure to sunlight, while others may display mutations linked to alkylating agents. Genomic contexts, such as chromatin structure and DNA accessibility, can also influence the efficiency of DNA repair processes, leading to variations in mutation patterns. Additionally, the fidelity of repair mechanisms may be compromised in specific genomic conditions, contributing to oncogenic mutagenesis. Understanding these variations is crucial for developing targeted therapies and personalized treatment strategies for different cancer types.
What are the potential therapeutic implications of understanding the strand-asymmetric mechanisms underlying DNA damage and repair in cancer?
Understanding the strand-asymmetric mechanisms underlying DNA damage and repair in cancer has significant therapeutic implications. Targeting specific repair pathways that exhibit strand-specific differences can potentially enhance the efficacy of cancer treatments. For instance, developing drugs that selectively inhibit translesion polymerases recruited to replication strands with persistent lesions could increase the sensitivity of cancer cells to DNA damage. Furthermore, identifying genomic conditions that compromise the fidelity of repair mechanisms can guide the development of novel therapeutic approaches aimed at restoring DNA repair efficiency. By leveraging this knowledge, researchers can design more effective and personalized treatment strategies that exploit the vulnerabilities of cancer cells while minimizing damage to normal tissues.
How can the insights from this study be leveraged to develop more accurate computational models of cancer genome evolution?
The insights from this study can be leveraged to develop more accurate computational models of cancer genome evolution by incorporating strand-asymmetric mechanisms of DNA damage and repair. By integrating data on strand-specific mutation patterns and repair processes, computational models can better simulate the dynamics of mutagenesis and clonal evolution in cancer. These models can take into account the influence of replication and transcription on DNA damage, as well as the impact of repair efficiency on mutation accumulation. Additionally, by considering the role of DNA accessibility and repair gradients in shaping mutation landscapes, computational models can more accurately predict the evolutionary trajectories of cancer genomes. Ultimately, these refined models can provide valuable insights into the mechanisms driving cancer progression and aid in the development of precision medicine approaches for cancer treatment.