innsikt - Computational Biology - # Structural Characterization of the Human Phosphate Exporter XPR1
Structural Insights into the Phosphate Export Mechanism of the Human XPR1 Transporter
Grunnleggende konsepter
The human XPR1 transporter plays a critical role in cellular phosphate homeostasis, and its dysfunction is linked to neurodegenerative diseases. This study provides structural insights into the gating and regulation mechanisms of XPR1-mediated phosphate efflux.
Sammendrag
The content discusses the structural and functional characterization of the human XPR1 transporter, which is the only known human phosphate (Pi) exporter and plays a crucial role in cellular Pi homeostasis. The key highlights are:
- XPR1 consists of an N-terminal SPX domain, a dimer-formation core domain, and a Pi transport domain.
- Within the transport domain, three basic clusters are responsible for Pi binding and transport, and a conserved W573 acts as a molecular switch for gating.
- The SPX domain binds to inositol polyphosphate (InsP6) and facilitates Pi efflux by liberating the C-terminal loop that limits Pi entry.
- The study provides a structural framework for understanding the mechanisms of Pi homeostasis regulation by XPR1 homologues in fungi, plants, and animals.
Tilpass sammendrag
Omskriv med AI
Generer sitater
Oversett kilde
Til et annet språk
Generer tankekart
fra kildeinnhold
Besøk kilde
www.nature.com
Human XPR1 structures reveal phosphate export mechanism - Nature
Statistikk
Inorganic phosphate (Pi) is a fundamental macronutrient for all living organisms, and the homeostasis of Pi is critical for numerous biological activities.
Dysfunction of XPR1 is associated with neurodegenerative diseases.
Sitater
"As the only known human Pi exporter to date, XPR1 has an indispensable role in cellular Pi homeostasis."
"This study provides a conceptual framework for the mechanistic understanding of Pi homeostasis by XPR1 homologues in fungi, plants and animals."
Viktige innsikter hentet fra
by Rui Yan,Huiw... klokken www.nature.com 08-21-2024
https://www.nature.com/articles/s41586-024-07852-9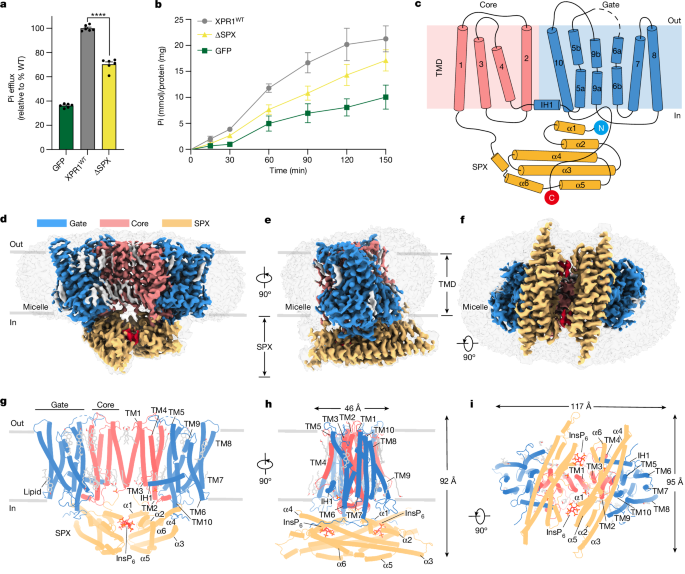
Dypere Spørsmål
How do the structural features of XPR1 enable it to maintain precise control over cellular phosphate levels?
The structural features of XPR1 play a crucial role in maintaining precise control over cellular phosphate levels. The protein consists of an N-terminal SPX domain, a dimer-formation core domain, and a Pi transport domain. Within the transport domain, three basic clusters are responsible for Pi binding and transport, ensuring efficient uptake and release of phosphate ions. Additionally, a conserved amino acid, W573, acts as a molecular switch for gating, allowing XPR1 to regulate the flow of phosphate ions in and out of the cell. The SPX domain binds to InsP6, a signaling molecule, and facilitates Pi efflux by releasing the C-terminal loop that restricts Pi entry. This intricate structural arrangement enables XPR1 to finely tune phosphate levels within the cell, essential for various biological activities.
What are the potential therapeutic implications of understanding the XPR1-mediated phosphate export mechanism for the treatment of neurodegenerative diseases?
Understanding the XPR1-mediated phosphate export mechanism holds significant therapeutic implications for the treatment of neurodegenerative diseases. Dysfunction of XPR1 has been linked to neurodegenerative conditions, highlighting the importance of maintaining proper cellular phosphate levels. By elucidating the structural basis of XPR1 gating and regulation by InsPPs, researchers can potentially develop targeted therapies to modulate XPR1 activity and restore phosphate homeostasis in affected individuals. This could help mitigate the progression of neurodegenerative diseases associated with XPR1 dysfunction, offering new avenues for treatment and management strategies.
What other cellular processes or signaling pathways might be influenced by the regulation of phosphate homeostasis through the XPR1 transporter?
The regulation of phosphate homeostasis through the XPR1 transporter can impact various cellular processes and signaling pathways beyond neurodegenerative diseases. Phosphate is essential for energy metabolism, DNA synthesis, and cell signaling, making its balance crucial for overall cellular function. Disruption in phosphate levels mediated by XPR1 could affect processes like ATP production, cell growth, and proliferation. Moreover, phosphate availability influences signaling pathways such as the mTOR pathway, which regulates cell growth and metabolism. By modulating phosphate transport through XPR1, the activation of these pathways could be altered, leading to broader implications for cellular physiology and potentially offering new targets for therapeutic interventions in various diseases.
0
