Comprehensive Single-cell Transcriptomic Atlas Reveals Cellular Vulnerabilities and Resilience in Alzheimer's Disease Across Brain Regions
Główne pojęcia
Comprehensive single-cell transcriptomic analysis of the aged human brain identifies vulnerable and resilient cell populations, region-specific pathways, and astrocyte programs associated with cognitive resilience in Alzheimer's disease.
Streszczenie
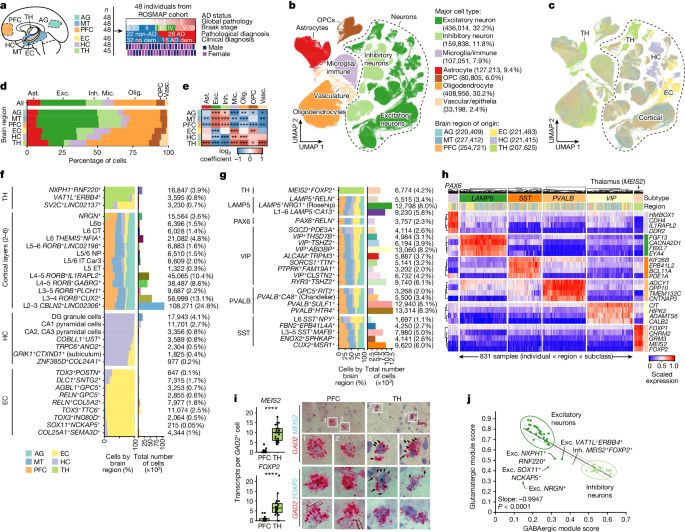
This study presents a comprehensive single-cell transcriptomic atlas of the aged human brain, covering 1.3 million cells from 283 post-mortem samples across 48 individuals with and without Alzheimer's disease. The key insights are:
-
Identification of 76 distinct cell types, including region-specific subtypes of astrocytes and excitatory neurons, and a unique inhibitory interneuron population in the thalamus.
-
Discovery of vulnerable populations of excitatory and inhibitory neurons that are depleted in specific brain regions in Alzheimer's disease, and evidence that the Reelin signaling pathway is involved in modulating the vulnerability of these neurons.
-
Development of a scalable method for identifying cell-type-specific and region-specific gene modules that are altered in Alzheimer's disease, providing insights into transcriptomic differences associated with diverse pathological variables.
-
Identification of an astrocyte program associated with cognitive resilience to Alzheimer's disease pathology, linking choline metabolism and polyamine biosynthesis in astrocytes to preserved cognitive function late in life.
The study provides a comprehensive regional atlas of the aging human brain and offers valuable insights into the cellular vulnerabilities, responses, and resilience mechanisms in Alzheimer's disease.
Przetłumacz źródło
Na inny język
Generuj mapę myśli
z treści źródłowej
Odwiedź źródło
www.nature.com
Single-cell multiregion dissection of Alzheimer’s disease - Nature
Statystyki
We analyzed 1.3 million cells from 283 post-mortem human brain samples across 48 individuals with and without Alzheimer's disease.
We identified 76 distinct cell types, including region-specific subtypes of astrocytes and excitatory neurons, and a unique inhibitory interneuron population in the thalamus.
Cytaty
"We identify vulnerable populations of excitatory and inhibitory neurons that are depleted in specific brain regions in Alzheimer's disease, and provide evidence that the Reelin signalling pathway is involved in modulating the vulnerability of these neurons."
"We identify an astrocyte program that is associated with cognitive resilience to Alzheimer's disease pathology, tying choline metabolism and polyamine biosynthesis in astrocytes to preserved cognitive function late in life."
Głębsze pytania
How do the identified vulnerable and resilient cell populations interact and influence each other in the progression of Alzheimer's disease?
In the context of Alzheimer's disease progression, the identified vulnerable and resilient cell populations play crucial roles in influencing each other. The vulnerable populations of excitatory and inhibitory neurons that are depleted in specific brain regions in Alzheimer's disease are likely to contribute to the cognitive decline and neurodegeneration observed in affected individuals. These neurons may show increased susceptibility to the toxic effects of amyloid-beta and tau proteins, leading to synaptic dysfunction and neuronal loss. On the other hand, the resilient cell populations, such as the astrocytes with a specific gene expression program associated with cognitive resilience, may provide neuroprotection and support cognitive function despite the presence of Alzheimer's pathology. These astrocytes could potentially modulate the inflammatory response, provide metabolic support to neurons, and maintain synaptic integrity, thereby promoting cognitive resilience in the face of neurodegeneration. The interaction between these vulnerable and resilient cell populations highlights the complex cellular dynamics underlying Alzheimer's disease progression and underscores the importance of understanding both the mechanisms of vulnerability and resilience in developing effective therapeutic strategies.
What are the potential therapeutic implications of targeting the Reelin signaling pathway or the astrocyte program associated with cognitive resilience?
Targeting the Reelin signaling pathway or the astrocyte program associated with cognitive resilience holds significant therapeutic implications for Alzheimer's disease. The Reelin signaling pathway, which has been implicated in modulating the vulnerability of excitatory and inhibitory neurons in specific brain regions, could be a promising target for therapeutic intervention. By enhancing Reelin signaling, it may be possible to protect vulnerable neurons from degeneration and promote their survival, thereby preserving cognitive function in Alzheimer's disease. Similarly, modulating the astrocyte program associated with cognitive resilience, particularly focusing on choline metabolism and polyamine biosynthesis, could offer a novel therapeutic approach. By promoting the neuroprotective functions of astrocytes, such as maintaining synaptic health and providing metabolic support to neurons, it may be possible to enhance cognitive resilience and slow down the progression of Alzheimer's pathology. Targeting these pathways could lead to the development of precision medicine approaches that aim to restore cellular homeostasis and promote brain health in individuals with Alzheimer's disease.
What other cellular pathways or molecular mechanisms beyond transcriptomics could contribute to the regional heterogeneity and differential vulnerability observed in Alzheimer's disease?
Beyond transcriptomics, several other cellular pathways and molecular mechanisms could contribute to the regional heterogeneity and the observed differential vulnerability in Alzheimer's disease. One such pathway is the immune response, particularly the activation of microglia and the release of pro-inflammatory cytokines, which play a critical role in neuroinflammation and neuronal damage in Alzheimer's disease. Dysregulation of synaptic plasticity and neurotransmitter systems, such as glutamatergic and GABAergic signaling, could also contribute to regional vulnerability by affecting synaptic function and neuronal communication. Additionally, alterations in mitochondrial function, oxidative stress, and protein misfolding pathways, including the ubiquitin-proteasome system and autophagy, may impact cellular health and contribute to neurodegeneration in specific brain regions. Epigenetic modifications, such as DNA methylation and histone acetylation, could further influence gene expression patterns and cellular responses to Alzheimer's pathology, adding another layer of complexity to regional heterogeneity. Understanding the interplay of these diverse cellular pathways and molecular mechanisms is essential for unraveling the full spectrum of factors contributing to the differential vulnerability observed in Alzheimer's disease and for developing comprehensive therapeutic strategies targeting multiple aspects of the disease process.