The FDA approved the Abrysvo vaccine for RSV in pregnant women, offering protection to infants. The vaccine is administered between weeks 32 and 36 of pregnancy. It aims to prevent lower respiratory tract illness caused by RSV in individuals aged 60 years and older. RSV is a common cause of illness in children, with infants at high risk for severe complications. The new vaccine has shown an 82% reduction in the risk of severe complications in clinical trials. RSV can lead to hospitalization, with infants and older individuals at the highest risk. The vaccine also aims to prevent severe RSV in infants under 1 year old. However, there are some reported side effects, including preeclampsia, low birth weight, and jaundice in infants of vaccinated pregnant individuals.
Dostosuj podsumowanie
Przepisz z AI
Generuj cytaty
Przetłumacz źródło
Na inny język
Generuj mapę myśli
z treści źródłowej
Odwiedź źródło
www.medscape.com
FDA Approves First RSV Vaccine for Pregnancy
Kluczowe wnioski z
by Lisa O'Mary o www.medscape.com 08-22-2023
https://www.medscape.com/viewarticle/995719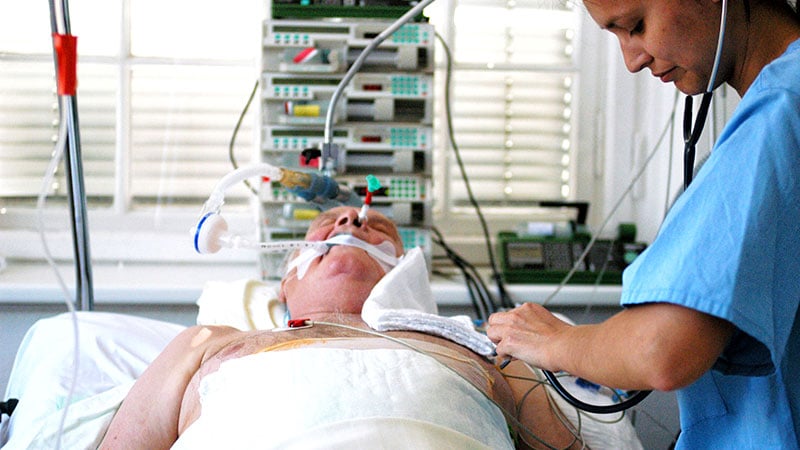
Głębsze pytania
