insight - Biomedical Engineering - # Spatiotemporal Modeling of Colorectal Oncogenesis in Engineered Mini-Colons
Spatiotemporally Controlled Colorectal Oncogenesis in Engineered Mini-Colons Enables Real-Time Tracking of Tumor Formation and Evolution
Conceitos essenciais
Engineered mini-colons that undergo tumorigenesis ex vivo enable spatiotemporally controlled induction and real-time tracking of colorectal cancer development at single-cell resolution.
Resumo
The content describes the development of a novel organoid-based system that allows for the spatiotemporally controlled induction and real-time tracking of colorectal cancer development. The key highlights are:
- Limitations of current 3D organoid culture technologies in capturing the intricate evolutionary process of cancer development in terms of tissue architecture, cell diversity, homeostasis, and lifespan.
- The authors developed "mini-colons" that integrate microfabrication, optogenetics, and tissue engineering approaches to enable tumorigenesis ex vivo.
- In this system, oncogenic transformation can be spatiotemporally controlled by directing oncogenic activation through blue-light exposure, and the emergent colon tumors can be tracked in real-time at single-cell resolution for several weeks without disrupting the culture.
- The induced mini-colons display rich intra-tumoral and inter-tumoral diversity, and recapitulate key pathophysiological hallmarks of colorectal tumors in vivo.
- By fine-tuning cell-intrinsic and cell-extrinsic parameters, the mini-colons can be used to identify tumorigenic determinants and pharmacological opportunities.
- This study paves the way for cancer initiation research outside living organisms, providing a powerful platform for spatiotemporal modeling of colorectal oncogenesis.
Personalizar Resumo
Reescrever com IA
Gerar Citações
Traduzir Fonte
Para outro idioma
Gerar Mapa Mental
do conteúdo fonte
Visitar Fonte
www.nature.com
Spatiotemporally resolved colorectal oncogenesis in mini-colons ex vivo - Nature
Estatísticas
Three-dimensional organoid culture technologies have revolutionized cancer research by allowing for more realistic and scalable reproductions of both tumour and microenvironmental structures.
Available organoid systems do not capture the intricate evolutionary process of cancer development in terms of tissue architecture, cell diversity, homeostasis and lifespan.
Oncogenesis and tumour formation studies are not possible in vitro and instead require the extensive use of animal models, which provide limited spatiotemporal resolution of cellular dynamics.
Citações
"As a consequence, oncogenesis and tumour formation studies are not possible in vitro and instead require the extensive use of animal models, which provide limited spatiotemporal resolution of cellular dynamics and come at a considerable cost in terms of resources and animal lives."
"By fine-tuning cell-intrinsic and cell-extrinsic parameters, mini-colons can be used to identify tumorigenic determinants and pharmacological opportunities."
Principais Insights Extraídos De
by L. F... às www.nature.com 04-24-2024
https://www.nature.com/articles/s41586-024-07330-2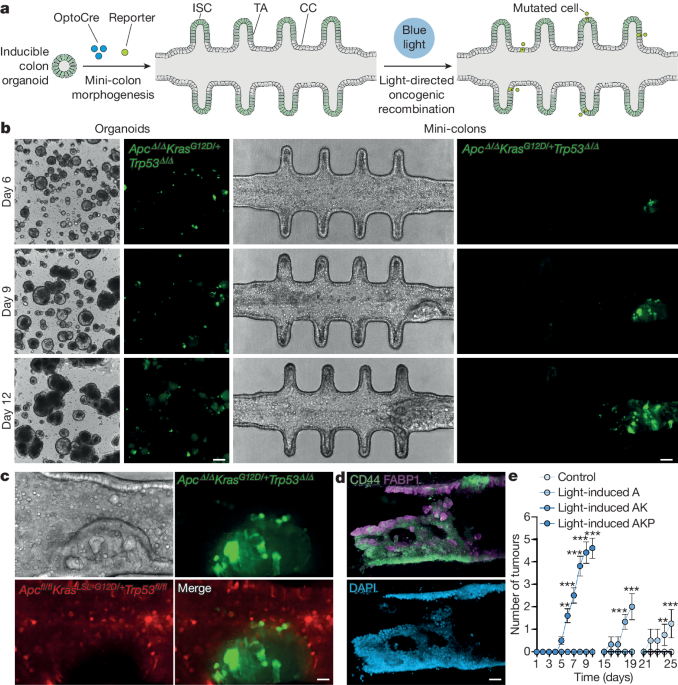
Perguntas Mais Profundas
How can the mini-colon system be further optimized to better recapitulate the complex tumor microenvironment and interactions with the immune system?
To better recapitulate the complex tumor microenvironment and interactions with the immune system, the mini-colon system can be optimized in several ways. Firstly, incorporating immune cells such as T cells, macrophages, and dendritic cells into the mini-colon culture can mimic the immune response seen in colorectal cancer. This can be achieved by co-culturing immune cells with mini-colons or by engineering the mini-colons to produce chemokines and cytokines that attract immune cells. Additionally, introducing components of the extracellular matrix (ECM) and stromal cells can enhance the structural complexity of the mini-colons, better mimicking the tumor microenvironment. By including fibroblasts, endothelial cells, and pericytes, the mini-colon system can more accurately represent the cellular interactions and signaling pathways present in colorectal tumors. Furthermore, incorporating microbiota or microbial components into the mini-colon system can provide insights into the role of the gut microbiome in colorectal cancer development and progression.
What are the potential limitations and challenges in translating the insights gained from the mini-colon system to clinical applications for colorectal cancer?
While the mini-colon system offers a powerful platform for studying colorectal cancer initiation and progression, there are several limitations and challenges in translating these insights to clinical applications. One major limitation is the lack of full recapitulation of the in vivo tumor microenvironment, as the mini-colons may not fully capture the complexity of interactions between tumor cells, immune cells, stromal cells, and the ECM. Additionally, the scalability and reproducibility of the mini-colon system may pose challenges in generating sufficient data for clinical applications. Furthermore, the cost and technical expertise required to maintain and analyze the mini-colon cultures may limit widespread adoption in clinical settings. Another challenge is the need to validate the findings from the mini-colon system in preclinical animal models and eventually in human clinical trials to ensure the relevance and efficacy of potential therapeutic interventions.
What other types of cancers or disease models could benefit from the development of similar spatiotemporally controlled organoid-based systems?
The development of similar spatiotemporally controlled organoid-based systems could benefit a wide range of cancers and disease models beyond colorectal cancer. For example, pancreatic cancer, which is known for its aggressive nature and poor prognosis, could greatly benefit from organoid systems that allow for the study of tumor initiation, progression, and response to therapy in a controlled environment. Similarly, breast cancer, lung cancer, and prostate cancer could benefit from organoid models that recapitulate the complex interactions between tumor cells, stromal cells, and the immune system. Moreover, rare cancers or pediatric cancers that are challenging to study in traditional cell culture systems could be better understood using organoid-based models. Additionally, chronic diseases such as inflammatory bowel disease, liver cirrhosis, and neurodegenerative disorders could benefit from spatiotemporally controlled organoid systems to investigate disease mechanisms and test potential therapeutics in a more physiologically relevant context.
0
