аналитика - Biotechnology - # Transferrin Receptor Targeting Chimeras (TransTACs) for Membrane Protein Degradation and Cancer Therapy
Transferrin Receptor Targeting Chimeras: A Novel Approach for Efficient Membrane Protein Degradation and Targeted Cancer Therapy
Основные понятия
Transferrin receptor targeting chimeras (TransTACs) can efficiently degrade a diverse range of membrane proteins, enabling reversible control of primary cells and targeting drug-resistant cancers.
Аннотация
The content describes the development and application of transferrin receptor targeting chimeras (TransTACs), a novel heterobispecific antibody modality for membrane protein degradation.
Key highlights:
- Cancer cells require high levels of iron for rapid proliferation, leading to significant upregulation of the cell-surface transferrin receptor 1 (TfR1), which mediates iron uptake.
- TransTACs are engineered to drive rapid co-internalization of a target protein of interest and TfR1 from the cell surface, and to enable target protein entry into the lysosomal degradation pathway.
- TransTACs can efficiently degrade a diverse range of single-pass, multi-pass, native or synthetic membrane proteins, including epidermal growth factor receptor, programmed cell death 1 ligand 1, cluster of differentiation 20, and chimeric antigen receptor.
- In example applications, TransTACs enabled the reversible control of human primary chimeric antigen receptor T cells and the targeting of drug-resistant epidermal growth factor receptor-driven lung cancer with the exon 19 deletion/T790M/C797S mutations in a mouse xenograft model.
- TransTACs represent a promising new family of bifunctional antibodies for precise manipulation of membrane proteins and targeted cancer therapy.
Настроить сводку
Переписать с помощью ИИ
Создать цитаты
Перевести источник
На другой язык
Создать интеллект-карту
из исходного контента
Перейти к источнику
www.nature.com
Transferrin receptor targeting chimeras for membrane protein degradation - Nature
Статистика
Cancer cells require high levels of iron for rapid proliferation.
Transferrin receptor 1 (TfR1) mediates iron uptake by binding to the iron-carrying protein transferrin.
TransTACs can efficiently degrade a diverse range of single-pass, multi-pass, native or synthetic membrane proteins.
Цитаты
"Leveraging this phenomenon and the fast endocytosis rate of TfR1 (refs. 4,5), we developed transferrin receptor targeting chimeras (TransTACs), a heterobispecific antibody modality for membrane protein degradation."
"TransTACs are engineered to drive rapid co-internalization of a target protein of interest and TfR1 from the cell surface, and to enable target protein entry into the lysosomal degradation pathway."
Ключевые выводы из
by Dingpeng Zha... в www.nature.com 09-25-2024
https://www.nature.com/articles/s41586-024-07947-3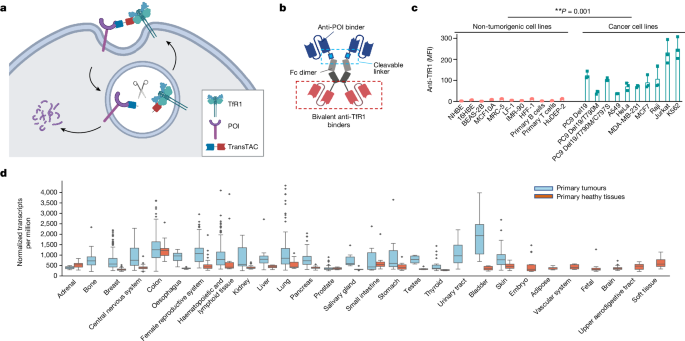
Дополнительные вопросы
How can TransTACs be further optimized to improve their efficiency and specificity in membrane protein degradation?
To enhance the efficiency and specificity of TransTACs in membrane protein degradation, several strategies can be employed. First, optimizing the binding affinity of the chimeric antibodies to the transferrin receptor 1 (TfR1) can improve the internalization rate of the target proteins. This could involve engineering the antibody fragments to increase their stability and binding kinetics, potentially through techniques such as affinity maturation or the incorporation of non-canonical amino acids to enhance interactions.
Second, the design of TransTACs can be refined to include additional targeting moieties that recognize specific epitopes on the target membrane proteins. This dual-targeting approach could minimize off-target effects and ensure that only the desired proteins are internalized and degraded. Additionally, incorporating elements that promote lysosomal trafficking, such as lysosomal targeting signals, could further enhance the degradation efficiency of the target proteins.
Third, the use of advanced delivery systems, such as nanoparticles or liposomes, could facilitate the targeted delivery of TransTACs to specific tissues or cell types, thereby increasing their therapeutic index. Finally, conducting comprehensive in vitro and in vivo studies to assess the pharmacokinetics and biodistribution of TransTACs will provide valuable insights for optimizing their design and application in targeted cancer therapy.
What are the potential off-target effects and safety concerns associated with the use of TransTACs in clinical settings?
The use of TransTACs in clinical settings raises several potential off-target effects and safety concerns. One primary concern is the possibility of unintended degradation of non-target membrane proteins that share structural similarities with the intended targets. This could lead to adverse effects on normal cellular functions and contribute to toxicity, particularly in healthy tissues that express the transferrin receptor 1 (TfR1).
Another safety concern is the immune response elicited by the chimeric antibodies. Patients may develop anti-drug antibodies (ADAs) against the TransTACs, which could neutralize their therapeutic effects or lead to hypersensitivity reactions. Additionally, the rapid internalization and degradation of target proteins could disrupt critical signaling pathways, potentially resulting in unintended consequences such as altered cell proliferation or apoptosis.
Furthermore, the long-term effects of TransTACs on the immune system and overall health are not yet fully understood. Rigorous preclinical and clinical studies are essential to evaluate the safety profile, including potential off-target effects, immunogenicity, and long-term consequences of using TransTACs in cancer therapy.
What other applications, beyond cancer therapy, could TransTACs have in the field of biotechnology and medicine?
Beyond cancer therapy, TransTACs hold significant potential for various applications in biotechnology and medicine. One promising area is the treatment of autoimmune diseases, where the targeted degradation of specific membrane proteins involved in immune responses could help modulate aberrant immune activity. For instance, TransTACs could be designed to selectively degrade pro-inflammatory receptors or ligands, thereby alleviating symptoms associated with conditions like rheumatoid arthritis or lupus.
Additionally, TransTACs could be utilized in the field of gene therapy. By targeting and degrading specific membrane proteins that inhibit gene delivery or expression, TransTACs could enhance the efficacy of viral or non-viral gene delivery systems. This could be particularly beneficial in treating genetic disorders where the restoration of functional proteins is required.
TransTACs may also find applications in the development of vaccines. By targeting and degrading specific receptors on antigen-presenting cells, TransTACs could enhance the presentation of vaccine antigens, leading to improved immune responses. Furthermore, their ability to manipulate membrane proteins could be harnessed in regenerative medicine, where the targeted degradation of inhibitory proteins could promote tissue repair and regeneration.
Overall, the versatility of TransTACs as a tool for precise manipulation of membrane proteins opens up numerous avenues for innovative therapeutic strategies across various fields of medicine and biotechnology.
0
