Mitochondrial Division as a Mechanism for Balancing Competing Metabolic Reactions
Основные понятия
Mitochondria, the powerhouses of cells, can divide themselves to separate competing metabolic reactions, ensuring the efficient production of necessary substrates for cellular function.
Аннотация
This short article highlights a research study published in Nature by Ryu et al. that investigated how mitochondria manage competing metabolic reactions. The researchers discovered that mitochondria can segregate themselves to support different metabolic processes. This division of labor allows mitochondria to optimize the production of essential substrates required for cellular functions. The study sheds light on the adaptive mechanisms of mitochondria in response to cellular energy demands.
Настроить сводку
Переписать с помощью ИИ
Создать цитаты
Перевести источник
На другой язык
Создать интеллект-карту
из исходного контента
Перейти к источнику
www.nature.com
Division of labour: mitochondria split to meet energy demands
Статистика
Цитаты
Ключевые выводы из
by Lena Pernas в www.nature.com 11-06-2024
https://www.nature.com/articles/d41586-024-03469-0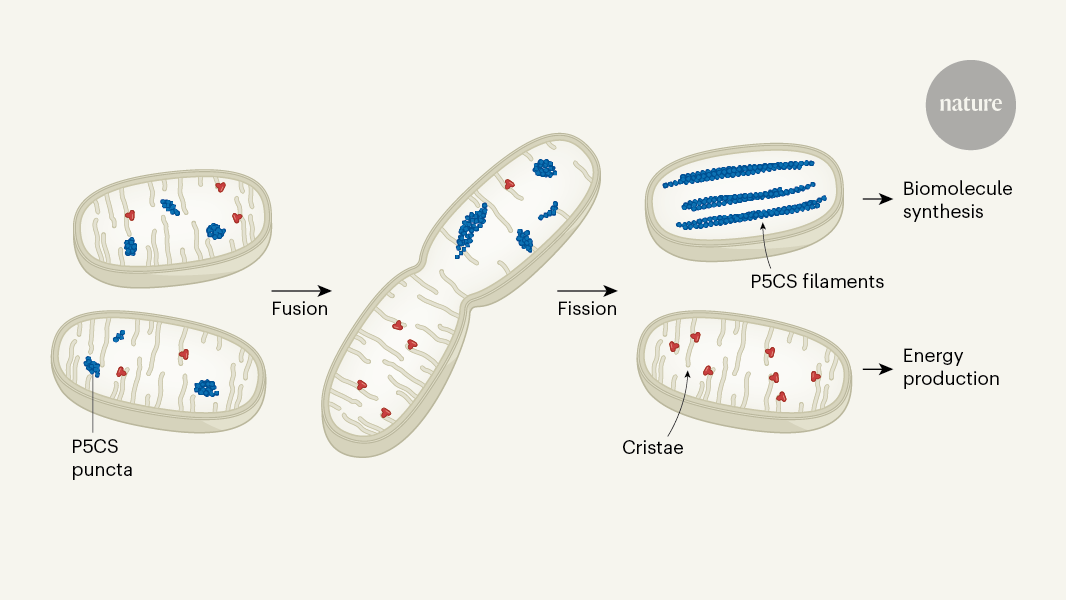
Дополнительные вопросы
How does the process of mitochondrial division relate to overall cellular health and disease?
Mitochondrial division, also known as mitochondrial fission, is crucial for maintaining overall cellular health. This process ensures that:
New cells receive a sufficient number of mitochondria: During cell division, mitochondrial fission allows for the proper distribution of mitochondria to daughter cells, guaranteeing they have the energy supply needed for growth and function.
Damaged mitochondria are removed: Fission facilitates the segregation and subsequent elimination of damaged mitochondrial components through mitophagy, a selective form of autophagy. This quality control mechanism prevents the accumulation of dysfunctional mitochondria, which can lead to oxidative stress and cellular damage.
Metabolic demands are met: As highlighted in the context, mitochondrial fission may play a role in partitioning different metabolic tasks, ensuring efficient energy production and biosynthesis.
Dysregulation of mitochondrial fission is implicated in various diseases, including:
Neurodegenerative disorders: Impaired fission is linked to Alzheimer's disease, Parkinson's disease, and Huntington's disease, where defective mitochondrial quality control and energy deficits contribute to neuronal dysfunction.
Cancer: Cancer cells often exhibit increased mitochondrial fission, which is thought to promote tumor growth, metastasis, and resistance to therapy.
Metabolic diseases: Disruptions in mitochondrial dynamics are associated with metabolic disorders like obesity, type 2 diabetes, and cardiovascular disease.
Therefore, maintaining a balance between mitochondrial fission and its counterpart, mitochondrial fusion, is essential for cellular health and preventing disease.
Could there be alternative mechanisms besides mitochondrial fission that contribute to managing competing metabolic reactions within the cell?
While mitochondrial fission offers a compelling mechanism for organizing metabolic processes, other cellular strategies likely contribute to managing competing reactions:
Substrate channeling: Enzymes responsible for sequential steps in a metabolic pathway can form complexes, allowing direct transfer of intermediates without diffusion into the bulk mitochondrial matrix. This minimizes competition for substrates and increases efficiency.
Spatial organization within mitochondria: Mitochondria possess distinct subcompartments, such as the outer membrane, intermembrane space, inner membrane, and matrix. Different metabolic pathways are localized to specific regions, creating microenvironments that optimize reaction conditions and limit substrate competition.
Allosteric regulation: Enzymes can be modulated by the binding of specific molecules, altering their activity and substrate affinity. This allows for fine-tuning of metabolic flux based on cellular needs and substrate availability.
Post-translational modifications: Reversible modifications like phosphorylation, acetylation, and ubiquitination can dynamically regulate enzyme activity and localization, influencing metabolic flux in response to cellular cues.
Transcriptional and translational control: The expression levels of enzymes involved in different metabolic pathways can be adjusted to match cellular demands, ensuring appropriate allocation of resources.
These mechanisms, in concert with mitochondrial fission, likely orchestrate a complex regulatory network that ensures efficient and adaptable cellular metabolism.
If we can control the division of mitochondria, can we potentially manipulate cellular energy production for therapeutic purposes?
The ability to control mitochondrial division holds significant therapeutic potential for various diseases:
Neurodegenerative diseases: Enhancing mitochondrial fission and mitophagy could help clear damaged mitochondria, improving neuronal function and potentially slowing disease progression.
Cancer: Inhibiting fission in cancer cells could limit their energy supply and proliferation, offering a novel therapeutic strategy.
Metabolic diseases: Modulating mitochondrial dynamics might improve metabolic efficiency and glucose homeostasis in conditions like obesity and type 2 diabetes.
Ischemia-reperfusion injury: Controlling mitochondrial fission and fusion during periods of oxygen deprivation and reperfusion could protect cells from damage and improve tissue recovery.
However, several challenges need to be addressed before realizing this therapeutic potential:
Specificity: Developing targeted therapies that specifically modulate mitochondrial fission without disrupting other cellular processes is crucial.
Delivery: Efficiently delivering therapeutic agents to the target cells and tissues remains a challenge.
Long-term effects: The long-term consequences of manipulating mitochondrial dynamics need to be carefully evaluated.
Despite these challenges, the prospect of manipulating mitochondrial division for therapeutic benefit represents an exciting frontier in biomedical research. Further research is needed to translate this knowledge into effective treatments for a wide range of diseases.
0
