Sustainable Bulk Alloy Production: Transforming Oxides into High-Performance Alloys through Innovative Solid-State Processing
The traditional metallurgical production process involves three sequential steps: extracting metals from ores, mixing them into alloys through liquid processing, and then thermomechanical processing to achieve the desired microstructures. However, this approach is not sustainable, as it accounts for almost 10% of all greenhouse gas emissions due to the use of fossil reductants and high-temperature processing.
The authors present a new H2-based redox synthesis and compaction approach that reforms the traditional alloy-making process. This method merges metal extraction, alloying, and thermomechanical processing into a single solid-state operation, unlocking tremendous opportunities for sustainable bulk alloy design.
The authors provide a thermodynamically informed guideline and a general kinetic conception to dissolve the classical boundaries between extractive and physical metallurgy. They exemplify this approach using the case of Fe-Ni invar bulk alloys, which are known to be eco-unfriendly due to the high CO2 emissions associated with Ni production.
The authors' sustainable method turns oxides directly into green alloys in bulk forms, with application-worthy properties, all obtained at temperatures far below the bulk melting point, while maintaining a zero CO2 footprint.
Настроить сводку
Переписать с помощью ИИ
Создать цитаты
Перевести источник
На другой язык
Создать интеллект-карту
из исходного контента
Перейти к источнику
www.nature.com
One step from oxides to sustainable bulk alloys - Nature
Ключевые выводы из
by Shaolou Wei,... в www.nature.com 09-18-2024
https://www.nature.com/articles/s41586-024-07932-w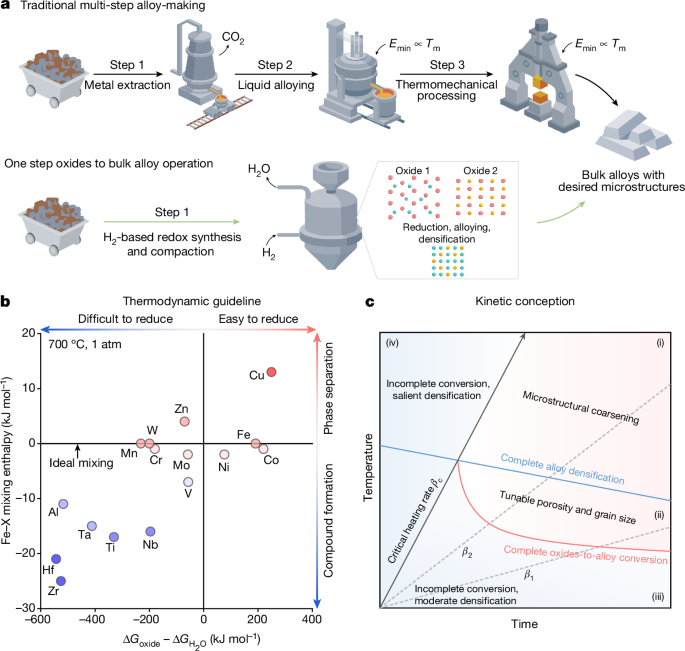
Дополнительные вопросы
