Risk Factors for Different Rheumatoid Arthritis-Associated Interstitial Lung Disease Subtypes Identified
The study examined the risk factors associated with different subtypes of rheumatoid arthritis-associated interstitial lung disease (RA-ILD) using data from two cohorts in the Mass General Brigham Healthcare system.
The researchers identified 208 patients with RA-ILD and 547 control participants with rheumatoid arthritis (RA) but no ILD. RA-ILD subtypes were determined using high-resolution computed tomography (HRCT) imaging.
The key findings are:
- The RA-UIP subtype, which has the worst prognosis, was associated with older age at RA diagnosis, male sex, and seropositivity.
- The RA-NSIP subtype was significantly associated only with seropositivity.
- Nonfibrotic ILDs were significantly associated with positive smoking status and seropositivity.
- The combination of male sex, seropositivity, and positive smoking status was associated with a nearly sevenfold increased risk for RA-UIP.
The authors suggest that RA-ILD subtypes may have distinct risk factor profiles, emphasizing the importance of understanding RA-ILD disease heterogeneity to inform screening and prognostication strategies.
Настроить сводку
Переписать с помощью ИИ
Создать цитаты
Перевести источник
На другой язык
Создать интеллект-карту
из исходного контента
Перейти к источнику
www.medscape.com
RA Lung Disease Subtypes Have Distinct Risk Factor Profiles
Ключевые выводы из
by Edited Javed... в www.medscape.com 09-26-2024
https://www.medscape.com/viewarticle/rheumatoid-arthritis-lung-disease-subtypes-have-distinct-2024a1000hhy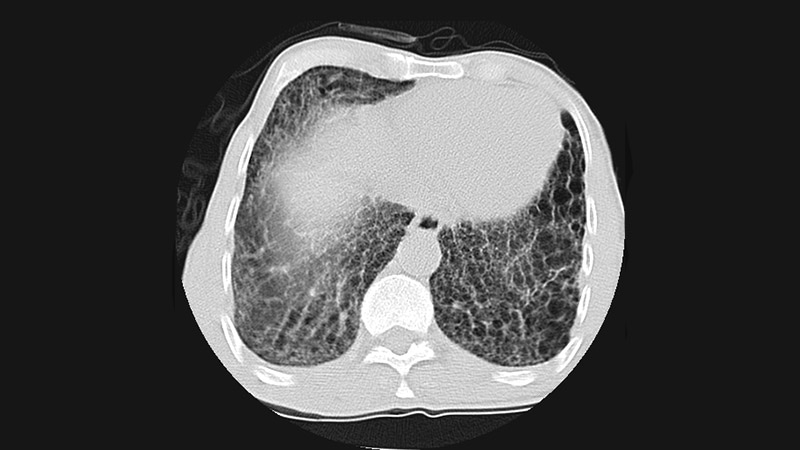
Дополнительные вопросы
