Precise Regulation of Transposon Methylation in Germline Development Requires Two-Factor Authentication in the piRNA Pathway
The PIWI-interacting RNA (piRNA) pathway guides the DNA methylation of young, active transposons during germline development in male mice. This process requires great precision, as every copy of the transposon needs to be methylated, but off-target methylation must be avoided.
The authors show that the chromatin reader SPIN1 interacts directly with SPOCD1, a key effector of piRNA-directed DNA methylation. SPIN1 expression precedes that of SPOCD1 and MIWI2, and young LINE1 copies are marked by H3K4me3, H3K9me3, and SPIN1 before piRNA-directed DNA methylation is initiated.
The authors generated a Spocd1 separation-of-function allele in mice, which encodes a SPOCD1 variant that no longer interacts with SPIN1. They found that the SPOCD1-SPIN1 interaction is essential for spermatogenesis and piRNA-directed DNA methylation of young LINE1 elements.
The authors propose that piRNA-directed LINE1 DNA methylation requires a two-factor authentication process: the first is the recruitment of SPIN1-SPOCD1 to the young LINE1 promoter, and the second is the engagement of MIWI2 with the nascent transcript. This independent authentication process ensures the precision of piRNA-directed transposon DNA methylation.
Настроить сводку
Переписать с помощью ИИ
Создать цитаты
Перевести источник
На другой язык
Создать интеллект-карту
из исходного контента
Перейти к источнику
www.nature.com
Two-factor authentication underpins the precision of the piRNA pathway - Nature
Ключевые выводы из
by Made... в www.nature.com 09-18-2024
https://www.nature.com/articles/s41586-024-07963-3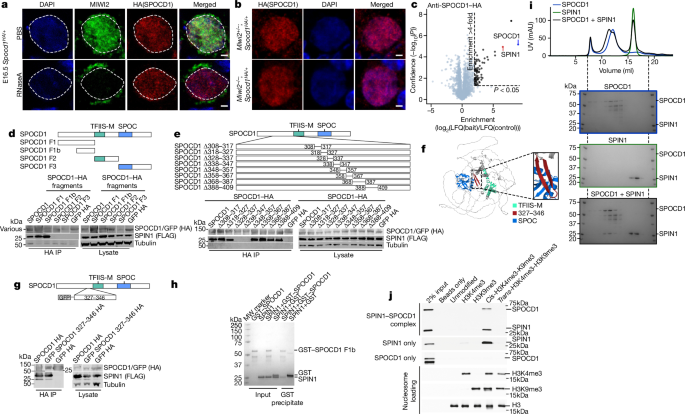
Дополнительные вопросы
