Discovery and Characterization of Broadly Inhibitory Antibodies Targeting Severe Malaria Virulence Proteins
This research paper details the discovery and characterization of two human monoclonal antibodies that exhibit broad reactivity against Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) variants associated with severe malaria.
Research Objective: The study aimed to investigate whether individual antibodies could recognize the diverse repertoire of circulating PfEMP1 variants and explore their potential as therapeutic or prophylactic agents against severe malaria.
Methodology: The researchers isolated two monoclonal antibodies from individuals and characterized their binding affinities to various CIDRα1 domains, representing different subclasses of PfEMP1. They employed biochemical assays to assess the antibodies' ability to inhibit EPCR binding of both recombinant and native PfEMP1 proteins. Additionally, they utilized bioengineered 3D human brain microvessels to evaluate the antibodies' efficacy in blocking parasite sequestration under physiologically relevant flow conditions. Structural analyses, including X-ray crystallography, were conducted to elucidate the molecular interactions between the antibodies and CIDRα1 variants.
Key Findings: The two antibodies demonstrated consistent and broad EPCR-binding inhibition across diverse CIDRα1 domains, encompassing five out of six subclasses. Both antibodies effectively inhibited the binding of both recombinant full-length and native PfEMP1 proteins to EPCR. Notably, the antibodies significantly impeded parasite sequestration in the brain microvessel model under flow conditions. Structural analyses revealed that both antibodies engaged with CIDRα1 through a conserved mechanism, targeting three highly conserved amino acid residues crucial for EPCR binding.
Main Conclusions: The study provides compelling evidence that broadly reactive antibodies targeting conserved epitopes within CIDRα1 can effectively neutralize the virulence of diverse PfEMP1 variants associated with severe malaria. These findings highlight the potential of these antibodies as promising candidates for developing novel therapeutics or vaccines against this deadly disease.
Significance: This research significantly advances our understanding of humoral immunity against severe malaria and offers a promising avenue for developing effective interventions. The identification of broadly inhibitory antibodies targeting conserved epitopes on PfEMP1 represents a significant step towards developing a vaccine or therapeutic strategy with broad efficacy against severe malaria.
Limitations and Future Research: Further research is warranted to evaluate the in vivo efficacy and safety of these antibodies in preclinical and clinical settings. Investigating the prevalence and functional diversity of these broadly reactive antibodies in malaria-exposed populations will provide valuable insights into naturally acquired immunity.
Özeti Özelleştir
Yapay Zeka ile Yeniden Yaz
Alıntıları Oluştur
Kaynağı Çevir
Başka Bir Dile
Zihin Haritası Oluştur
kaynak içeriğinden
Kaynak
www.nature.com
Broadly inhibitory antibodies to severe malaria virulence proteins - Nature
Önemli Bilgiler Şuradan Elde Edildi
by Raphael A. R... : www.nature.com 11-20-2024
https://www.nature.com/articles/s41586-024-08220-3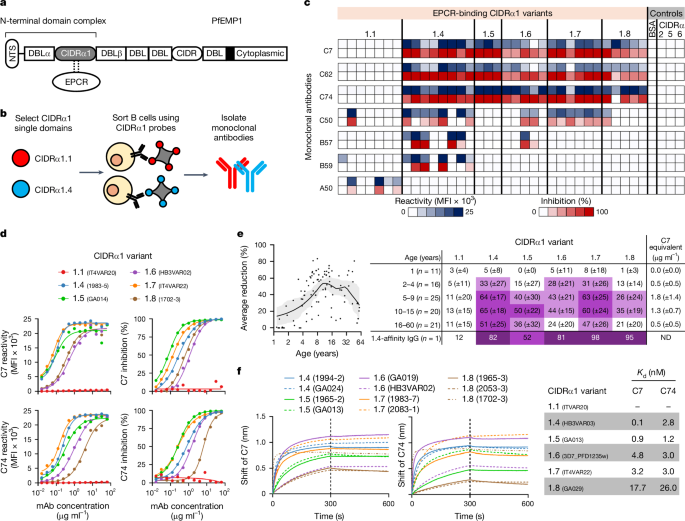
Daha Derin Sorular
