洞見 - Computational Biology - # Impact of Fasting and Refeeding on Intestinal Stem Cells and Tumorigenesis
Post-Fast Refeeding Enhances Intestinal Stem Cell Proliferation and Tumor Formation
核心概念
Post-fast refeeding increases intestinal stem cell proliferation and tumor formation by enhancing mTORC1-driven protein synthesis through polyamine metabolism.
摘要
The article explores the effects of fasting and post-fast refeeding on adult stem cells and tumor formation, specifically in the context of the intestinal stem cells (ISCs). The key findings are:
- Post-fast refeeding increases ISC proliferation and tumor formation compared to the fasted or ad libitum-fed states.
- Post-fast refeeding augments the regenerative capacity of Lgr5+ ISCs.
- Loss of the tumor suppressor gene Apc in post-fast-refed ISCs leads to a higher tumor incidence in the small intestine and colon.
- Mechanistically, robust mTORC1 induction in post-fast-refed ISCs increases protein synthesis via polyamine metabolism, driving the observed changes in regeneration and tumorigenicity.
- Inhibition of mTORC1, polyamine metabolite production, or protein synthesis abrogates the regenerative or tumorigenic effects of post-fast refeeding.
The authors conclude that fast-refeeding cycles must be carefully considered and tested when planning diet-based strategies for regeneration, as post-fast refeeding leads to a burst in stem-cell-driven regeneration and tumorigenicity.
客製化摘要
使用 AI 重寫
產生引用格式
翻譯原文
翻譯成其他語言
產生心智圖
從原文內容
前往原文
www.nature.com
Short-term post-fast refeeding enhances intestinal stemness via polyamines - Nature
統計資料
Post-fast refeeding leads to a higher tumor incidence in the small intestine and colon compared to the fasted or ad libitum-fed states.
Inhibition of mTORC1, polyamine metabolite production, or protein synthesis abrogates the regenerative or tumorigenic effects of post-fast refeeding.
引述
"Post-fast refeeding augments the regenerative capacity of Lgr5+ ISCs, and loss of the tumour suppressor gene Apc in post-fast-refed ISCs leads to a higher tumour incidence in the small intestine and colon than in the fasted or ad libitum-fed states, demonstrating that post-fast refeeding is a distinct state."
"Mechanistically, we discovered that robust mTORC1 induction in post-fast-refed ISCs increases protein synthesis via polyamine metabolism to drive these changes, as inhibition of mTORC1, polyamine metabolite production or protein synthesis abrogates the regenerative or tumorigenic effects of post-fast refeeding."
從以下內容提煉的關鍵洞見
by Shin... 於 www.nature.com 08-21-2024
https://www.nature.com/articles/s41586-024-07840-z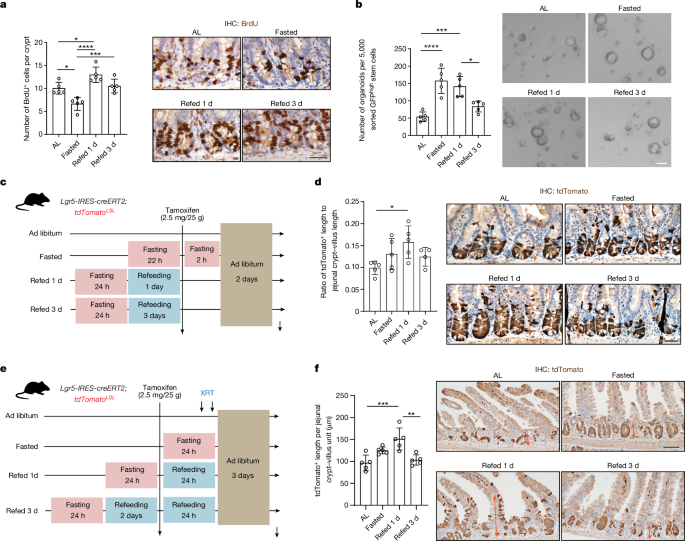
深入探究
What are the potential long-term health implications of repeated fast-refeeding cycles, and how can these be mitigated?
Repeated fast-refeeding cycles can have potential long-term health implications, especially in terms of increasing the risk of tumorigenesis. The study highlights that post-fast refeeding leads to a burst in stem-cell-driven regeneration and tumorigenicity, particularly in the intestines. This heightened regenerative capacity of intestinal stem cells (ISCs) post-refeeding can potentially lead to an increased incidence of tumors in the small intestine and colon, especially when combined with the loss of the tumor suppressor gene Apc. Therefore, a key concern is the balance between promoting regeneration and inadvertently increasing cancer risk through these cycles.
To mitigate these potential health implications, careful consideration and testing of fast-refeeding cycles are essential when planning diet-based strategies for regeneration. One approach could involve monitoring the frequency and duration of fast-refeeding cycles to prevent excessive stimulation of ISCs and tumorigenesis. Additionally, targeting specific molecular pathways involved in the regenerative and tumorigenic effects of post-fast refeeding, such as mTORC1 signaling and polyamine metabolism, could offer potential therapeutic interventions to modulate these processes and reduce the associated health risks.
How do the effects of post-fast refeeding on intestinal stem cells and tumorigenesis differ across various age groups or genetic backgrounds?
The effects of post-fast refeeding on intestinal stem cells (ISCs) and tumorigenesis may vary across different age groups and genetic backgrounds due to variations in cellular responses and molecular pathways. Age-related changes in stem cell function and regenerative capacity could influence the outcomes of post-fast refeeding on ISCs. Older individuals may exhibit alterations in ISC proliferation and differentiation, which could impact the regenerative potential and tumorigenicity of ISCs post-refeeding.
Moreover, genetic backgrounds play a crucial role in determining the susceptibility to tumorigenesis and the response of ISCs to post-fast refeeding. Genetic mutations, such as the loss of the tumor suppressor gene Apc as demonstrated in the study, can significantly affect the tumorigenic potential of ISCs following refeeding. Different genetic backgrounds may modulate the activity of key signaling pathways involved in ISC regulation, thereby influencing the regenerative capacity and tumorigenic potential of ISCs in response to post-fast refeeding.
Understanding these age- and genetic-related differences in the effects of post-fast refeeding on ISCs and tumorigenesis is essential for personalized approaches to dietary interventions and cancer prevention strategies.
Could the insights from this study be leveraged to develop targeted therapies for intestinal diseases or cancers?
The insights from this study regarding the effects of post-fast refeeding on intestinal stem cells (ISCs) and tumorigenesis offer valuable opportunities for the development of targeted therapies for intestinal diseases and cancers. By elucidating the molecular mechanisms underlying the regenerative and tumorigenic effects of post-fast refeeding, researchers can identify potential therapeutic targets for intervention.
Targeting key pathways such as mTORC1 signaling and polyamine metabolism, which drive the regenerative and tumorigenic responses of ISCs post-refeeding, could lead to the development of novel therapeutic strategies. Inhibiting mTORC1 activation or disrupting polyamine metabolism in ISCs post-refeeding may help prevent excessive stem cell proliferation and tumorigenesis in the intestines.
Furthermore, the identification of specific genetic mutations, such as the loss of the tumor suppressor gene Apc, as critical factors in promoting tumorigenesis post-refeeding, could guide the development of precision medicine approaches for individuals with these genetic alterations. By leveraging the insights from this study, targeted therapies that modulate ISC function and prevent tumorigenesis could be developed to improve the treatment outcomes of intestinal diseases and cancers.
0
