Distinct Neuronal Ensembles Mediate Positive and Negative Reinforcement Effects of Fentanyl
核心概念
Distinct populations of µ-opioid receptor-expressing neurons in the ventral tegmental area and central amygdala mediate the positive and negative reinforcement effects of fentanyl, respectively.
摘要
The article investigates the neuronal mechanisms underlying the positive and negative reinforcement effects of the powerful opioid painkiller fentanyl. Fentanyl use can lead to addiction, which is driven by both positive reinforcement (euphoria) and negative reinforcement (avoidance of withdrawal symptoms).
The key findings are:
- Acute fentanyl administration inhibits GABAergic neurons in the ventral tegmental area (VTA), leading to disinhibition of dopamine neurons and increased dopamine release in the nucleus accumbens, which mediates the positive reinforcement effects.
- Knockdown of µ-opioid receptors in the VTA abolished the dopamine transients and positive reinforcement, but did not affect the withdrawal symptoms.
- Neurons expressing µ-opioid receptors in the central amygdala (CeA) showed enhanced activity during fentanyl withdrawal, and knockdown of these receptors eliminated the aversive withdrawal symptoms, suggesting they mediate the negative reinforcement.
- Optogenetic stimulation of the CeA neurons expressing µ-opioid receptors caused place aversion, and mice learned to self-administer to pause this stimulation.
The study delineates the distinct neuronal populations and circuits in the VTA and CeA that underlie the positive and negative reinforcement effects of fentanyl, providing insights for developing interventions to reduce addiction and facilitate rehabilitation.
客製化摘要
使用 AI 重寫
產生引用格式
翻譯原文
翻譯成其他語言
產生心智圖
從原文內容
前往原文
www.nature.com
Distinct µ-opioid ensembles trigger positive and negative fentanyl reinforcement - Nature
統計資料
Fentanyl use leads to addiction in one-fourth of users, the largest fraction for all addictive drugs.
引述
"Fentanyl is a powerful painkiller that elicits euphoria and positive reinforcement1. Fentanyl also leads to dependence, defined by the aversive withdrawal syndrome, which fuels negative reinforcement2,3 (that is, individuals retake the drug to avoid withdrawal)."
"Knockdown of µ-opioid receptors in VTA abolished dopamine transients and positive reinforcement, but withdrawal remained unchanged."
"We identified neurons expressing µ-opioid receptors in the central amygdala (CeA) whose activity was enhanced during withdrawal. Knockdown of µ-opioid receptors in CeA eliminated aversive symptoms, suggesting that they mediate negative reinforcement."
從以下內容提煉的關鍵洞見
by Fabr... 於 www.nature.com 05-22-2024
https://www.nature.com/articles/s41586-024-07440-x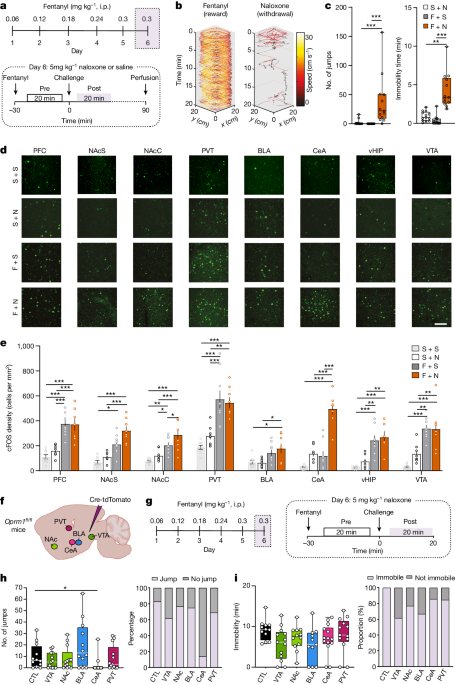
深入探究
How do the neuronal circuits identified in this study interact with other brain regions involved in addiction, such as the prefrontal cortex or the hippocampus?
The neuronal circuits identified in this study, particularly those involving the ventral tegmental area (VTA) and the central amygdala (CeA), interact with other brain regions implicated in addiction, such as the prefrontal cortex and the hippocampus. The VTA is known to project dopaminergic neurons to the nucleus accumbens, a key component of the brain's reward system. This interaction plays a crucial role in the positive reinforcement associated with fentanyl use. On the other hand, the CeA, which is involved in emotional processing and stress responses, interacts with regions like the prefrontal cortex and hippocampus to mediate negative reinforcement during withdrawal. These interactions likely contribute to the complex interplay of positive and negative reinforcement mechanisms in fentanyl addiction.
What are the potential limitations of the mouse model used in this study, and how can the findings be validated in human subjects?
One potential limitation of using a mouse model is the inherent biological differences between mice and humans, which may not fully capture the complexity of human addiction. Mice may not exhibit the same behavioral responses or neural adaptations to fentanyl as humans do. Additionally, the dosage of fentanyl administered to mice may not accurately reflect human usage patterns. To validate the findings in human subjects, translational research involving clinical studies or human brain imaging techniques could be employed. This would allow researchers to observe similar neural circuitry changes in human brains exposed to fentanyl and confirm the relevance of the identified circuits in human addiction.
Could the insights from this study be leveraged to develop novel pharmacological or neuromodulation-based interventions for treating fentanyl addiction and withdrawal?
The insights from this study offer promising avenues for developing novel interventions to treat fentanyl addiction and withdrawal. By targeting specific neuronal populations in the VTA and CeA that are involved in positive and negative reinforcement, researchers could potentially design pharmacological agents that modulate µ-opioid receptors or other key molecules to disrupt these circuits. Additionally, neuromodulation techniques such as deep brain stimulation or transcranial magnetic stimulation could be explored to directly modulate the activity of these circuits and alleviate addiction-related behaviors. By understanding the precise neural mechanisms underlying fentanyl addiction, tailored interventions could be developed to address both the rewarding and aversive aspects of the addiction cycle.
0
