Discovery of Hybrid Protein Filaments in Neurodegenerative Diseases Challenges Previous Understanding
The content describes a surprising new finding in the field of neurodegenerative diseases. Traditionally, these diseases have been defined by the aggregation of protein filaments made from single types of proteins, such as tau, α-synuclein or amyloid-β. However, a recent study published in Nature has identified the first disease-associated filament composed of two distinct proteins - TAR DNA-binding protein 43 (TDP-43) and annexin A11 (ANXA11).
Using cryo-electron microscopy (cryo-EM), the researchers were able to show how fragments from these two proteins interact to assemble into helical filaments. This discovery provides new insights into the mechanistic origins of these disease-causing entities, challenging the previous understanding of neurodegenerative diseases.
The study represents a significant advancement in the field, as it suggests that the formation of protein filaments in neurodegenerative diseases may be more complex than previously thought, involving the interaction of multiple proteins. This finding could have important implications for the development of new diagnostic and therapeutic approaches targeting these hybrid protein filaments.
Налаштувати зведення
Переписати за допомогою ШІ
Згенерувати цитати
Перекласти джерело
Іншою мовою
Згенерувати інтелект-карту
із вихідного контенту
Перейти до джерела
www.nature.com
Hybrid protein filaments are a surprise twist in neurodegeneration
Ключові висновки, отримані з
by Michael S. F... о www.nature.com 10-02-2024
https://www.nature.com/articles/d41586-024-03054-5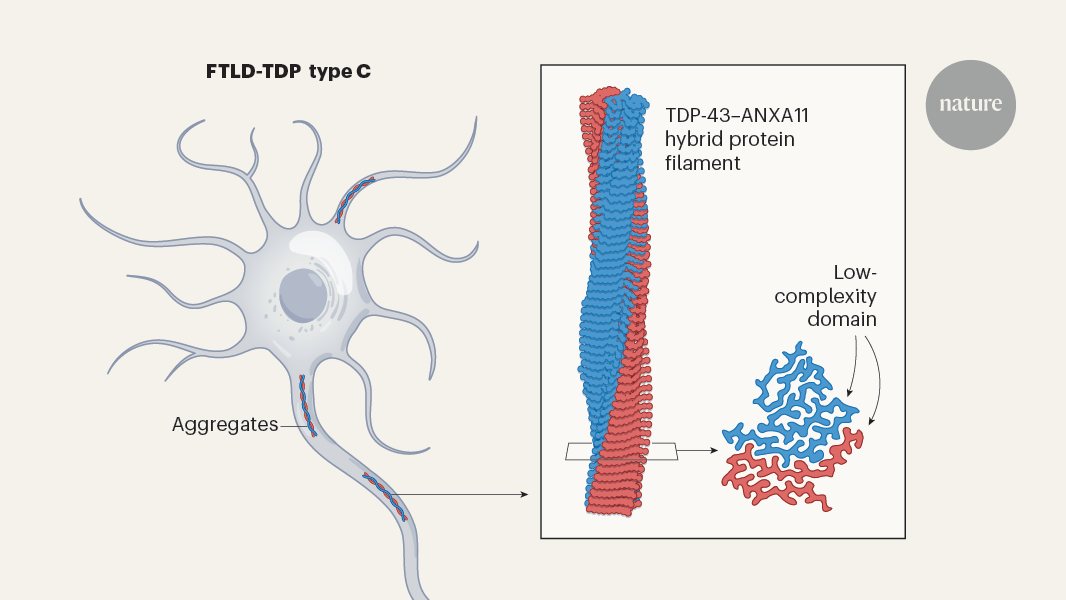
Глибші Запити
