Catalytic Enantioselective Synthesis of Meta Benzene Isosteres for Improved Pharmaceutical Properties
The article discusses the limitations of aromatic rings in pharmaceutical compounds and the potential benefits of replacing them with non-aromatic isosteric motifs. Aromatic rings can lead to suboptimal potency, metabolic stability, solubility, and lipophilicity, while their planar and achiral nature may not fit well with the chiral binding pockets of many pharmaceutical targets.
The authors present a novel palladium-catalyzed reaction that converts hydrocarbon-derived precursors into chiral boron-containing nortricyclanes, which they propose as plausible isosteres for meta-disubstituted aromatic rings. This catalytic enantioselective reaction provides access to a broad array of nortricyclane structures, which can then be further transformed.
The authors demonstrate that incorporating nortricyclanes into pharmaceutical motifs can result in improved biophysical properties, as well as stereochemistry-dependent activity. They anticipate that the simple and inexpensive synthesis of the functionalized nortricyclane scaffold will make this platform a useful foundation for the assembly of new biologically active agents.
Налаштувати зведення
Переписати за допомогою ШІ
Згенерувати цитати
Перекласти джерело
Іншою мовою
Згенерувати інтелект-карту
із вихідного контенту
Перейти до джерела
www.nature.com
Catalytic asymmetric synthesis of meta benzene isosteres - Nature
Ключові висновки, отримані з
by Mingkai Zhan... о www.nature.com 08-21-2024
https://www.nature.com/articles/s41586-024-07865-4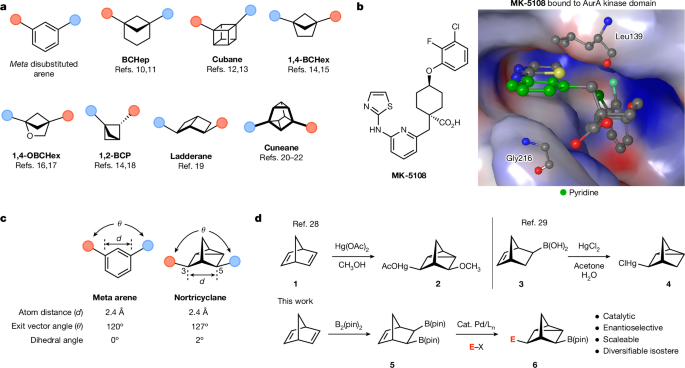
Глибші Запити
