Intermediate Rb-E2F Activity State Regulates Cell Proliferation Commitment
Conceitos Básicos
Cells maintain an intermediate Rb-E2F activity state before committing to proliferation, allowing them to sense and respond to various signals.
Resumo
The article explores the mechanism by which mammalian cells regulate the decision between quiescence and proliferation. It focuses on the role of the positive feedback loop between the transcription factor E2F and the cyclin-dependent kinase CDK2.
The key findings are:
Cells spend variable and often extended times in a reversible state of intermediate E2F activity before committing to proliferate.
This intermediate E2F activity is proportional to the amount of phosphorylation of a conserved T373 residue in the Rb protein, which is mediated by CDK2 or CDK4/CDK6.
The T373-phosphorylated Rb remains bound to chromatin, but dissociates once Rb is hyperphosphorylated at many sites, fully activating E2F.
The preferential initial phosphorylation of T373 can be explained by its relatively slower rate of dephosphorylation.
The authors propose that this intermediate Rb-E2F activity state allows cells to sense external and internal signals and decide whether to reverse and exit to quiescence or trigger the positive feedback mechanism that initiates cell proliferation.
An intermediate Rb–E2F activity state safeguards proliferation commitment - Nature
Estatísticas
Cells spend variable and often extended times in a reversible state of intermediate E2F activity before committing to proliferate.
The intermediate E2F activity is proportional to the amount of phosphorylation of a conserved T373 residue in Rb.
The T373-phosphorylated Rb remains bound on chromatin, but dissociates once Rb is hyperphosphorylated at many sites.
The preferential initial phosphorylation of T373 can be explained by its relatively slower rate of dephosphorylation.
Citações
"Cells spend variable and often extended times in a reversible state of intermediate E2F activity before committing to proliferate."
"The intermediate E2F activity is proportional to the amount of phosphorylation of a conserved T373 residue in Rb."
"The T373-phosphorylated Rb remains bound on chromatin, but dissociates once Rb is hyperphosphorylated at many sites, which fully activates E2F."
Principais Insights Extraídos De
by Yumi Konagay... às www.nature.com 06-26-2024
https://www.nature.com/articles/s41586-024-07554-2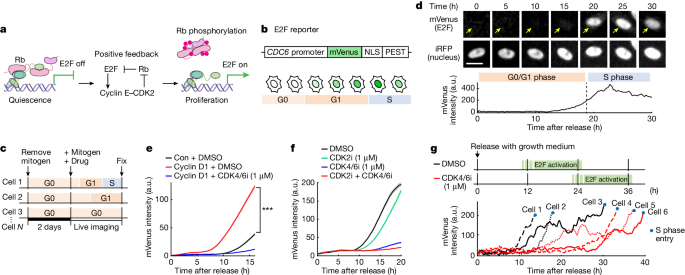
Perguntas Mais Profundas
How do external and internal signals influence the duration and dynamics of the intermediate Rb-E2F activity state?
The duration and dynamics of the intermediate Rb-E2F activity state are influenced by both external and internal signals. External signals, such as growth factors or stress stimuli, can trigger signaling pathways that converge on the Rb-E2F axis. These signals can modulate the activity of CDK2 and CDK4/CDK6, which phosphorylate the T373 residue on Rb, leading to the primed state of intermediate E2F activation. The timing and strength of these external signals can determine whether cells remain in the intermediate state or progress towards proliferation. Internal signals, including the levels of various cell cycle regulators and the cellular metabolic state, also play a crucial role in regulating the duration of the intermediate state. Cells integrate these signals to assess their readiness for proliferation, balancing the decision to either exit to quiescence or initiate cell cycle progression.
What are the potential implications of this intermediate state for the regulation of other cell fate decisions beyond proliferation?
The existence of an intermediate Rb-E2F activity state has significant implications for the regulation of other cell fate decisions beyond proliferation. This primed state allows cells to carefully assess their environment and internal conditions before committing to proliferation. The ability to pause at this intermediate stage provides cells with a window of opportunity to respond to changing signals or stresses. For example, cells could use this state to repair DNA damage, differentiate into specific cell types, or undergo apoptosis if necessary. By maintaining a reversible state of intermediate E2F activity, cells can make informed decisions about their fate, ensuring proper tissue homeostasis and function. Understanding the regulatory mechanisms governing this intermediate state could shed light on how cells coordinate various cellular processes beyond proliferation.
How might the insights from this study on the Rb-E2F pathway be leveraged to develop novel therapeutic strategies for diseases like cancer?
The insights gained from studying the Rb-E2F pathway offer promising avenues for developing novel therapeutic strategies for diseases like cancer. Dysregulation of the cell cycle, particularly aberrant activation of E2F transcription factors, is a hallmark of cancer. Targeting the intermediate Rb-E2F activity state could provide a unique opportunity to intervene in cancer progression. For instance, modulating the phosphorylation status of the T373 residue on Rb, which controls the transition from the intermediate to fully activated E2F state, could be a potential therapeutic target. By manipulating the signaling pathways that regulate this phosphorylation event, it may be possible to tip the balance towards quiescence or proliferation in cancer cells. Additionally, understanding the factors that influence the duration of the intermediate state could help in designing strategies to induce cell cycle arrest or promote apoptosis in cancer cells. Overall, leveraging the insights from this study could lead to the development of targeted therapies that specifically disrupt the proliferation commitment of cancer cells, offering new approaches for cancer treatment.
0
