Innovative Approach to Building Polycyclic Rings from Diradicals
Conceitos Básicos
The author presents a novel couple-close method for constructing semisaturated ring systems from dual radical precursors, addressing the limitations of current synthetic methods and enabling the creation of diverse saturated ring types with potential pharmaceutical applications.
Resumo
Heteroarenes offer advantages over arene counterparts due to their solubility properties and higher sp3 carbon content. Current synthetic methods for semisaturated heterocycles are limited, hindering their inclusion in drug development. The new approach described combines metallaphotoredox coupling with radical cyclization to efficiently produce various saturated ring structures with potential for late-stage functionalization in pharmaceutical synthesis.
Customize Summary
Rewrite with AI
Generate Citations
Translate Source
To Another Language
Generate MindMap
from source content
Visit Source
www.nature.com
Couple-close construction of polycyclic rings from diradicals - Nature
Estatísticas
Semisaturated heteroarenes possess a higher fraction of sp3 carbons.
Semisaturated heterocycles are challenging to prepare using current methods.
The new approach allows for the rapid assembly of diverse saturated ring types.
Reagent-controlled radical generation leads to highly regioselective annulation.
Citações
"Herein, we describe a more intuitive and modular couple-close approach to build semisaturated ring systems from dual radical precursors."
"The broad availability of the requisite feedstock materials allows sampling of regions of underexplored chemical space."
Principais Insights Extraídos De
by Alice Long,C... às www.nature.com 03-13-2024
https://www.nature.com/articles/s41586-024-07181-x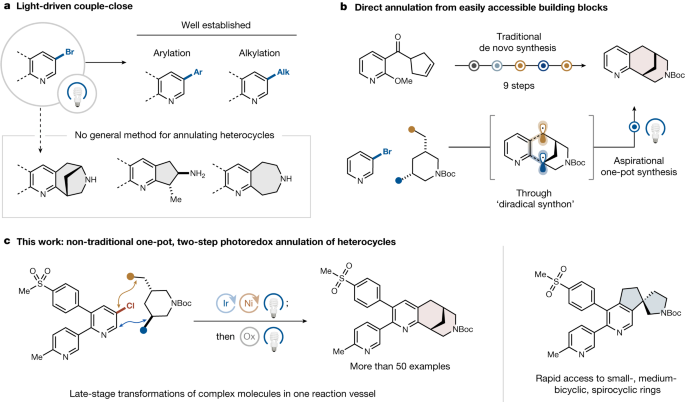
Perguntas Mais Profundas
How might this innovative method impact the development of new pharmaceutical compounds?
The innovative couple-close construction of polycyclic rings from diradicals presents a significant advancement in synthetic methodology that could revolutionize the development of new pharmaceutical compounds. By enabling the rapid assembly of semisaturated ring systems with desirable properties such as improved solubility, binding affinity, and specificity, this approach opens up avenues for the creation of novel drug candidates. The ability to efficiently access diverse spirocyclic, bridged, and substituted saturated ring types that were previously challenging to synthesize allows for exploration into uncharted chemical space. This method also facilitates late-stage functionalization of pharmaceutical scaffolds, offering a more streamlined route for modifying lead compounds and accelerating hit-to-lead campaigns in drug discovery.
What challenges could arise when implementing this approach on a larger scale in industrial settings?
While the couple-close strategy offers promising benefits for pharmaceutical compound development, several challenges may arise when scaling up this approach in industrial settings. One potential challenge is ensuring reproducibility and scalability of the reaction conditions across different batch sizes to maintain high yields and purity levels consistently. Additionally, optimizing process efficiency while minimizing waste generation becomes crucial when transitioning from laboratory-scale synthesis to large-scale production. Controlling costs associated with sourcing raw materials and reagents at bulk quantities without compromising quality poses another obstacle that needs careful consideration. Furthermore, addressing safety concerns related to handling reactive intermediates or byproducts on an industrial scale requires robust risk management protocols to ensure worker safety and regulatory compliance.
How can advancements in synthetic chemistry like this contribute to sustainable drug discovery practices?
Advancements in synthetic chemistry such as the couple-close construction method hold great potential for contributing to sustainable drug discovery practices through several key mechanisms. Firstly, by streamlining synthetic routes and reducing reliance on laborious fit-for-purpose syntheses, these innovative methods can enhance overall process efficiency and productivity within drug discovery pipelines. This increased efficiency translates into shorter development timelines and reduced resource consumption during compound optimization phases. Moreover, the ability to access structurally diverse yet pharmaceutically relevant molecules using modular approaches promotes chemical diversity screening efforts essential for identifying lead compounds with improved pharmacological profiles while minimizing environmental impact through fewer reaction steps and reduced waste generation.
0
