insight - Computational Biology - # Pathogenic Hypothalamic Extracellular Matrix and Metabolic Disease
Extracellular Matrix Remodeling in the Hypothalamus Contributes to Metabolic Disorders
Conceitos Básicos
Remodeling of the specialized extracellular matrix, termed perineuronal net, surrounding neurons in the arcuate nucleus of the hypothalamus drives insulin resistance and metabolic dysfunction in obesity and type 2 diabetes.
Resumo
The content discusses the role of the extracellular matrix (ECM) in the arcuate nucleus of the hypothalamus (ARC) in the development of metabolic diseases, such as obesity and type 2 diabetes.
Key highlights:
In metabolic disease, the perineuronal net (a specialized chondroitin sulfate proteoglycan ECM) surrounding ARC neurons becomes augmented and remodeled.
This remodeling of the ARC extracellular matrix drives insulin resistance and metabolic dysfunction.
Disrupting the perineuronal net, either enzymatically or with small molecules, in obese mice improves insulin access to the brain, reverses neuronal insulin resistance, and enhances metabolic health.
The findings identify ARC extracellular matrix remodeling as a fundamental mechanism driving metabolic diseases.
Pathogenic hypothalamic extracellular matrix promotes metabolic disease - Nature
Estatísticas
Metabolic diseases such as obesity and type 2 diabetes are marked by insulin resistance.
Cells within the arcuate nucleus of the hypothalamus (ARC), which are crucial for regulating metabolism, become insulin resistant during the progression of metabolic disease.
Citações
"In metabolic disease, the perineuronal net of the ARC becomes augmented and remodelled, driving insulin resistance and metabolic dysfunction."
"Disruption of the perineuronal net in obese mice, either enzymatically or with small molecules, improves insulin access to the brain, reversing neuronal insulin resistance and enhancing metabolic health."
Principais Insights Extraídos De
by Cait A. Bedd... às www.nature.com 09-18-2024
https://www.nature.com/articles/s41586-024-07922-y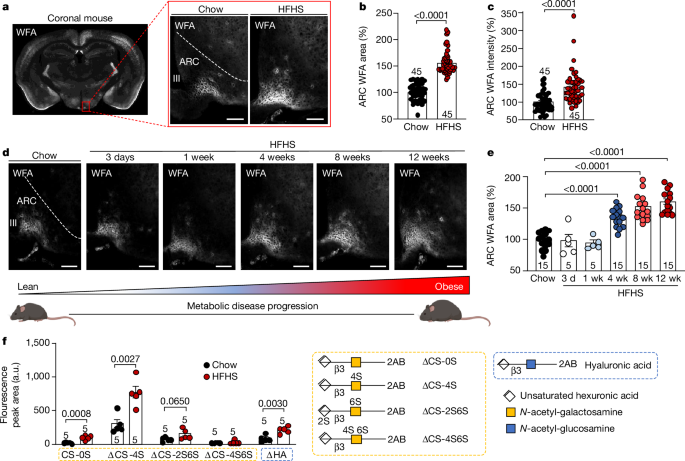
Perguntas Mais Profundas
What are the specific molecular mechanisms by which the remodeling of the perineuronal net in the ARC leads to insulin resistance and metabolic dysfunction?
The remodeling of the perineuronal net (PNN) in the arcuate nucleus of the hypothalamus (ARC) is a critical factor contributing to insulin resistance and metabolic dysfunction. The PNN is composed of specialized chondroitin sulfate proteoglycans that provide structural support to neurons. In the context of metabolic disease, the PNN undergoes augmentation and remodeling, which alters its composition and integrity. This remodeling can lead to the following molecular mechanisms:
Barrier Formation: The augmented PNN may create a physical barrier that impedes insulin access to ARC neurons. Insulin signaling is crucial for metabolic regulation, and restricted access can lead to diminished insulin receptor activation, resulting in insulin resistance.
Altered Signaling Pathways: The changes in the PNN can affect the signaling pathways within ARC neurons. For instance, the remodeling may disrupt the normal interaction between insulin receptors and downstream signaling molecules, such as PI3K and Akt, which are essential for glucose uptake and metabolism.
Inflammatory Responses: The remodeling of the PNN may also be associated with increased neuroinflammation, which is known to contribute to insulin resistance. Pro-inflammatory cytokines can alter neuronal function and exacerbate metabolic dysfunction.
Neuronal Plasticity: The structural changes in the PNN can affect neuronal plasticity, leading to impaired synaptic function and communication between neurons that regulate energy balance, further promoting metabolic dysregulation.
Overall, the remodeling of the PNN in the ARC creates a hostile environment for insulin signaling, leading to systemic metabolic dysfunction and insulin resistance.
How do the findings on the role of the ARC extracellular matrix in metabolic diseases compare to the involvement of other brain regions or peripheral tissues in the development of these disorders?
The findings regarding the role of the ARC extracellular matrix (ECM) in metabolic diseases highlight a specific and critical mechanism that differs from the involvement of other brain regions and peripheral tissues.
ARC vs. Other Brain Regions: The ARC is uniquely positioned to integrate metabolic signals and regulate energy homeostasis. While other brain regions, such as the ventromedial hypothalamus (VMH) and the lateral hypothalamus (LH), also play roles in energy balance, the specific remodeling of the PNN in the ARC appears to be a distinct mechanism that directly influences insulin signaling. Other regions may be involved in broader aspects of appetite regulation and energy expenditure but may not exhibit the same ECM remodeling associated with insulin resistance.
Peripheral Tissues: In peripheral tissues, such as adipose tissue and liver, insulin resistance is often linked to inflammation, lipid accumulation, and altered signaling pathways. While these tissues also contribute to metabolic diseases, the ARC's ECM remodeling represents a central nervous system-specific mechanism that directly impacts the brain's ability to respond to insulin. This suggests that targeting the ARC may provide a unique therapeutic avenue that complements strategies aimed at peripheral tissues.
Integration of Signals: The ARC serves as a hub for integrating peripheral signals (like leptin and ghrelin) and central signals (like insulin), making its ECM remodeling particularly impactful. In contrast, peripheral tissues primarily respond to local metabolic cues without the same level of integration seen in the brain.
In summary, while other brain regions and peripheral tissues are involved in the development of metabolic diseases, the specific remodeling of the ARC's ECM represents a unique and critical mechanism that directly influences insulin resistance and metabolic dysfunction.
Could targeting the extracellular matrix remodeling in the ARC be a potential therapeutic strategy for treating metabolic diseases, and what are the challenges and considerations in developing such interventions?
Targeting the remodeling of the extracellular matrix (ECM) in the arcuate nucleus of the hypothalamus (ARC) presents a promising therapeutic strategy for treating metabolic diseases, particularly insulin resistance and obesity. However, several challenges and considerations must be addressed in developing such interventions:
Mechanism of Action: Understanding the precise molecular mechanisms by which ECM remodeling contributes to insulin resistance is crucial. Therapeutic strategies must be designed to specifically target the components of the PNN that are altered in metabolic disease without disrupting normal neuronal function.
Delivery Methods: Effective delivery of therapeutic agents to the ARC is a significant challenge. The blood-brain barrier (BBB) limits the accessibility of many drugs, necessitating the development of novel delivery systems, such as nanoparticles or intranasal administration, to ensure that treatments reach the target neurons.
Potential Side Effects: Interventions aimed at modifying the ECM could have unintended consequences on neuronal health and function. Careful consideration must be given to the potential side effects, including impacts on synaptic plasticity and overall brain function.
Individual Variability: Metabolic diseases are heterogeneous, and individual responses to ECM-targeting therapies may vary. Personalized approaches may be necessary to optimize treatment efficacy based on specific metabolic profiles.
Long-term Effects: The long-term effects of ECM remodeling interventions on metabolic health and brain function need to be thoroughly investigated. Chronic alterations to the ECM could lead to unforeseen consequences, necessitating long-term studies to assess safety and efficacy.
Regulatory Considerations: Developing therapies that target the ECM will require navigating complex regulatory pathways to ensure safety and efficacy. This includes preclinical studies, clinical trials, and post-market surveillance.
In conclusion, while targeting ECM remodeling in the ARC holds potential as a therapeutic strategy for metabolic diseases, addressing these challenges and considerations is essential for the successful development and implementation of such interventions.
0
