How Gut Microbes Prevent Harmful Bacteria Invasion by Depriving Them of Nutrients
Conceitos Básicos
Gut commensal bacteria suppress colonization by harmful pathogens like Klebsiella pneumoniae through nutrient depletion.
Resumo
The article discusses how the gut microbiota, the community of microbes living in the intestines, plays a crucial role in maintaining colonization resistance against harmful bacterial invaders. When the diversity of the gut microbiota is compromised, often due to antibiotic treatment, it can enable the proliferation of potentially pathogenic species like Klebsiella pneumoniae and other Enterobacteriaceae.
The key insights are:
Membership in the gut microbiota is highly exclusive, with new arrivals typically rejected through a process called colonization resistance.
Loss of microbiotal diversity, usually caused by antibiotics, weakens colonization resistance and allows harmful bacteria to invade the gut and reach high densities.
The study by Furuichi et al. reveals how certain commensal bacteria normally present in the gut can suppress the colonization of K. pneumoniae by depriving it of essential nutrients.
Re-establishing a diverse and healthy gut microbiome is an important clinical goal to restore colonization resistance and prevent the overgrowth of harmful pathogens.
Gut microbes fend off harmful bacteria by depriving them of nutrients
Estatísticas
Antibiotic treatment can compromise microbiotal diversity and enable potentially harmful species like Klebsiella pneumoniae to invade the gut and reach high densities.
Citações
"Loss of microbiotal diversity, commonly the result of antibiotic treatment, compromises colonization resistance and enables potentially harmful species, such as the bacterial pathogen Klebsiella pneumoniae and other species belonging to the Enterobacteriaceae family, to invade the gut and reach high densities."
"Re-establishing colonization resistance is therefore an important clinical goal."
Principais Insights Extraídos De
by Eric G. Pame... às www.nature.com 09-18-2024
https://www.nature.com/articles/d41586-024-02803-w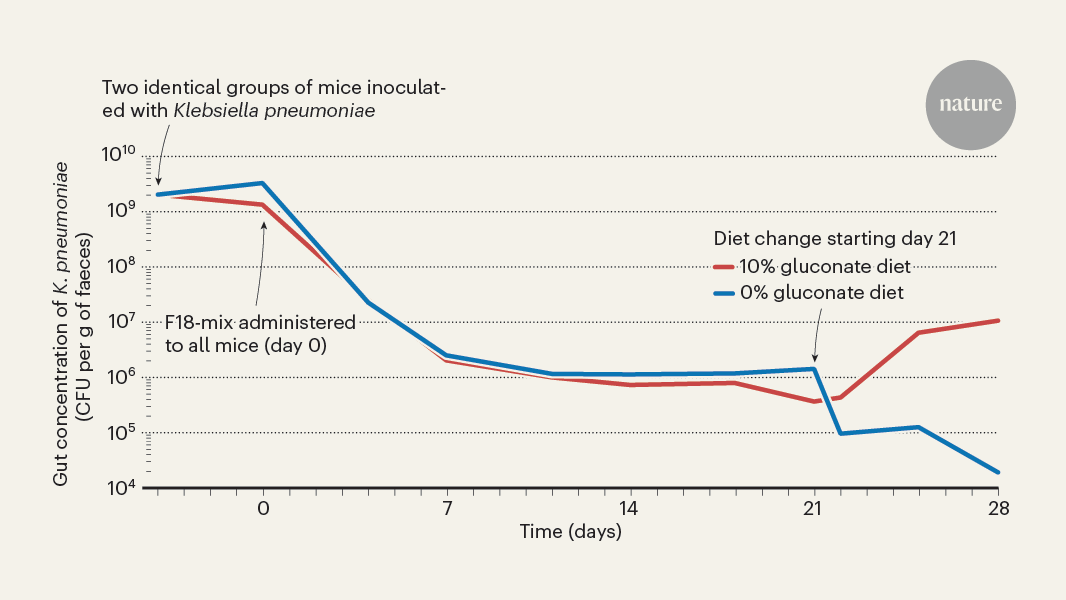
Perguntas Mais Profundas
What are the specific mechanisms by which commensal gut bacteria deprive harmful pathogens of essential nutrients to suppress their colonization?
Commensal gut bacteria employ several mechanisms to deprive harmful pathogens, such as Klebsiella pneumoniae, of essential nutrients, thereby suppressing their colonization. One primary mechanism is competitive exclusion, where beneficial microbes occupy niches in the gut, effectively outcompeting pathogens for limited resources. This competition can involve the consumption of carbohydrates, amino acids, and other nutrients that pathogens require for growth.
Additionally, commensal bacteria can produce metabolites, such as short-chain fatty acids (SCFAs), which not only serve as energy sources for intestinal cells but also create an unfavorable environment for pathogenic bacteria. These metabolites can lower the pH of the gut, inhibiting the growth of certain pathogens. Furthermore, some commensal species can secrete antimicrobial compounds, such as bacteriocins, which directly inhibit the growth of harmful bacteria. By utilizing these mechanisms, the gut microbiota maintains a delicate balance that is crucial for preventing pathogen invasion and promoting overall gut health.
How can we leverage the gut microbiome's colonization resistance capabilities to develop more effective therapies against antibiotic-resistant infections?
Leveraging the gut microbiome's colonization resistance capabilities presents a promising avenue for developing therapies against antibiotic-resistant infections. One approach is the use of probiotics, which are live beneficial bacteria that can be administered to restore or enhance the diversity and functionality of the gut microbiota. By introducing specific strains of commensal bacteria known to exhibit competitive exclusion or antimicrobial properties, we can potentially re-establish colonization resistance against harmful pathogens.
Another strategy involves fecal microbiota transplantation (FMT), where fecal matter from a healthy donor is transferred to a patient. This procedure aims to restore a diverse and balanced microbiome, which can help in combating antibiotic-resistant infections by reinstating the natural barriers against pathogen colonization. Additionally, researchers are exploring the development of prebiotics—substances that promote the growth of beneficial bacteria—as a means to enhance the gut microbiome's resilience. By focusing on these microbiome-based therapies, we can create innovative solutions to tackle the growing challenge of antibiotic resistance.
What other factors, beyond antibiotic use, can lead to a loss of microbiotal diversity and compromise the gut's ability to resist pathogen invasion?
Several factors beyond antibiotic use can contribute to a loss of microbiotal diversity, compromising the gut's ability to resist pathogen invasion. Diet plays a significant role; a diet high in processed foods, sugars, and unhealthy fats can negatively impact the composition of the gut microbiome, leading to reduced diversity. Conversely, a diet rich in fiber, fruits, and vegetables supports a diverse microbiota.
Lifestyle factors, such as stress, lack of sleep, and sedentary behavior, can also influence gut health. Chronic stress, for instance, can alter gut motility and increase intestinal permeability, allowing pathogens easier access to the gut. Environmental factors, including exposure to pollutants and chemicals, can disrupt the microbiome as well. Additionally, certain medical conditions, such as inflammatory bowel disease (IBD) or obesity, can lead to dysbiosis, further diminishing microbial diversity. Understanding these factors is crucial for developing strategies to maintain a healthy gut microbiome and enhance colonization resistance against harmful pathogens.
0
