Discovery and Characterization of Lolamicin: A Gram-Negative-Selective Antibiotic that Preserves the Gut Microbiome
Kernekoncepter
Lolamicin is a newly discovered antibiotic that selectively targets Gram-negative pathogens while sparing the gut microbiome, addressing the critical need for antibiotics that can treat infections without disrupting the commensal bacterial community.
Resumé
This article reports the design and discovery of a novel antibiotic called lolamicin that exhibits selective activity against Gram-negative bacteria over Gram-positive bacteria, as well as against pathogenic Gram-negative bacteria over commensal Gram-negative bacteria.
The key highlights and insights are:
Gram-negative infections are increasingly prevalent but are typically treated with broad-spectrum antibiotics, leading to disruption of the gut microbiome and susceptibility to secondary infections.
Lolamicin was designed to target the lipoprotein transport system, a pathway that is conserved in pathogenic Gram-negative bacteria but has low sequence homology in commensal Gram-negative bacteria.
Lolamicin demonstrated potent activity against a panel of more than 130 multidrug-resistant Gram-negative clinical isolates.
In mouse models of acute pneumonia and septicemia, lolamicin showed efficacy in treating the Gram-negative infections.
Importantly, lolamicin was able to spare the gut microbiome in mice, preventing secondary infection with Clostridioides difficile, a common consequence of broad-spectrum antibiotic use.
The selective killing of pathogenic Gram-negative bacteria by lolamicin is attributed to the low sequence homology of the target in pathogenic bacteria versus commensal bacteria, demonstrating a strategy that can be applied to develop other microbiome-sparing antibiotics.
A Gram-negative-selective antibiotic that spares the gut microbiome - Nature
Statistik
Lolamicin showed activity against a panel of more than 130 multidrug-resistant Gram-negative clinical isolates.
Lolamicin was effective in treating acute pneumonia and septicemia infections in mouse models.
Lolamicin prevented secondary Clostridioides difficile infection in mice, indicating preservation of the gut microbiome.
Citater
"There is a critical need for antibiotics that are selective both for Gram-negative bacteria over Gram-positive bacteria, as well as for pathogenic bacteria over commensal bacteria."
"The selective killing of pathogenic Gram-negative bacteria by lolamicin is a consequence of low sequence homology for the target in pathogenic bacteria versus commensals; this doubly selective strategy can be a blueprint for the development of other microbiome-sparing antibiotics."
Vigtigste indsigter udtrukket fra
by Kris... kl. www.nature.com 05-29-2024
https://www.nature.com/articles/s41586-024-07502-0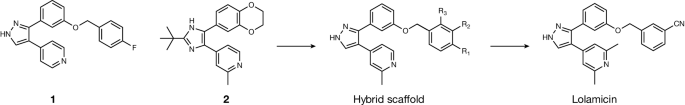
Dybere Forespørgsler
How can the design principles used to develop lolamicin be applied to target other pathogenic bacteria while preserving the beneficial commensal microbiome?
The design principles employed in developing lolamicin, a Gram-negative-specific antibiotic, can serve as a valuable framework for targeting other pathogenic bacteria while safeguarding the beneficial commensal microbiome. One key aspect is the focus on targeting specific bacterial components or pathways that are essential for the survival of pathogenic bacteria but are distinct or less critical in commensal bacteria. By identifying unique targets with low sequence homology between pathogenic and commensal bacteria, antibiotics can be designed to selectively disrupt the pathogen while sparing the beneficial microbiota. This approach ensures that the antibiotic's mechanism of action is tailored to the specific vulnerabilities of the pathogen, minimizing collateral damage to the commensal microbiome. Additionally, leveraging advanced screening techniques and computational modeling to identify novel targets specific to pathogenic bacteria can further enhance the development of antibiotics with selective activity, akin to lolamicin.
What are the potential limitations or drawbacks of the selective targeting approach used in lolamicin, and how can they be addressed?
While the selective targeting approach exemplified by lolamicin offers promising benefits in combating Gram-negative infections while preserving the gut microbiome, there are potential limitations and drawbacks that need to be considered. One significant challenge is the potential for the development of resistance mechanisms by pathogenic bacteria against the selective antibiotic. Pathogens may evolve to alter the target site or develop alternative pathways to bypass the antibiotic's mode of action, leading to reduced efficacy over time. To address this, a combination therapy approach involving multiple antibiotics with different mechanisms of action can be explored to mitigate the risk of resistance emergence. Additionally, continuous surveillance for resistance patterns and the development of strategies to overcome resistance, such as drug modification or synergistic drug combinations, are essential to prolong the effectiveness of selective antibiotics like lolamicin.
Given the increasing prevalence of Gram-negative infections, what other strategies or technologies are being explored to combat these pathogens without disrupting the gut microbiome?
In response to the rising incidence of Gram-negative infections and the imperative to preserve the gut microbiome, researchers are exploring various strategies and technologies to combat these pathogens effectively. One approach involves the development of narrow-spectrum antibiotics that specifically target essential pathways unique to Gram-negative bacteria, similar to the mechanism of lolamicin. By focusing on specific vulnerabilities of Gram-negative pathogens, these antibiotics can selectively eradicate the infection-causing bacteria while minimizing harm to the commensal microbiome. Furthermore, the advancement of precision medicine techniques, such as phage therapy and CRISPR-based antimicrobials, offers targeted and personalized treatment options for Gram-negative infections. These innovative technologies allow for precise targeting of pathogenic bacteria, reducing the impact on the gut microbiome and promoting the restoration of microbial balance. Additionally, the exploration of immunotherapies and probiotics as adjunct treatments to enhance host immunity and restore microbiome diversity represents a holistic approach to combatting Gram-negative infections while preserving gut health.
0
