Información - Biomedical Engineering - # Soft Biocompatible Implants for Continuous Glucose Monitoring
Soft Biocompatible Cubes Enable Continuous Glucose Monitoring Through Skin-Surface Implants
Conceptos Básicos
Soft biocompatible materials can help overcome the immune response and performance issues of rigid implanted glucose sensors, enabling continuous monitoring through skin-surface devices.
Resumen
The article discusses the challenges of designing effective implantable glucose sensors for continuous monitoring in diabetes patients. Traditionally, these sensors have faced issues due to the body's immune response, which forms a fibrous shell around the rigid electronic components, affecting the sensor's performance.
The authors propose a novel approach using soft, biocompatible materials that allow the harder sensor components to be positioned on the surface of the skin. This helps mitigate the immune response, as the softer material better matches the surrounding tissues. The key highlights are:
Continuous glucose monitoring is crucial for managing diabetes, but implantable sensors have proven difficult to design due to the body's immune response.
The immune system forms a fibrous shell around the rigid electronic components of implanted sensors, which can impact their performance.
The authors suggest using soft, biocompatible materials to house the sensor components, positioning them on the skin surface rather than implanting them.
This approach helps overcome the immune response issues by better matching the stiffness of the surrounding tissues.
The soft, squishy cubes used in the proposed method could enable effective continuous glucose monitoring through skin-surface devices.
Brain fluid probed by ultrasound using squishy cubes
Estadísticas
None
Citas
None
Ideas clave extraídas de
by Jules J. Mag... a las www.nature.com 06-05-2024
https://www.nature.com/articles/d41586-024-01423-8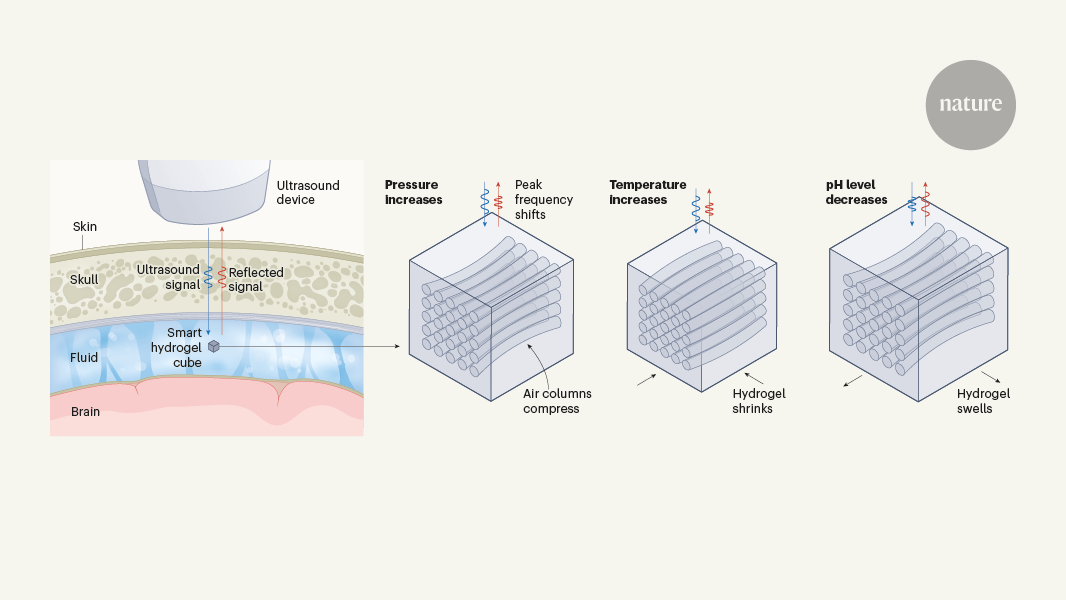
Consultas más profundas
How can the soft, biocompatible materials used in this approach be further optimized to improve the performance and longevity of the continuous glucose monitoring devices?
The soft, biocompatible materials used in this approach can be further optimized through several strategies. Firstly, researchers can focus on enhancing the biocompatibility of these materials to reduce the risk of immune response and fibrous encapsulation around the implanted devices. This can involve refining the surface properties of the materials to make them more compatible with the surrounding tissues. Additionally, improving the mechanical properties of the soft materials can help in reducing the mismatch with the body's natural flexibility, thereby minimizing the formation of fibrous capsules. Furthermore, optimizing the degradation rate of these materials to match the required lifespan of the monitoring devices can also enhance their performance and longevity. By fine-tuning these aspects, the soft, biocompatible materials can be tailored to create more effective and durable continuous glucose monitoring devices.
What other types of implantable medical devices could benefit from this soft material approach to reduce immune response and improve integration with the body?
The soft material approach used to reduce immune response and improve integration with the body can benefit various other types of implantable medical devices. For instance, neural implants, such as brain-computer interfaces or deep brain stimulators, could benefit from this approach to minimize the inflammatory response and enhance long-term functionality. Similarly, cardiac implants like pacemakers or defibrillators could utilize soft, biocompatible materials to improve biointegration and reduce the risk of adverse reactions. Moreover, drug delivery systems, such as insulin pumps or chemotherapy devices, could also benefit from this approach to enhance patient comfort and treatment efficacy. By applying the soft material strategy to a wide range of implantable medical devices, it is possible to improve their performance, longevity, and overall compatibility with the body.
What are the potential challenges or limitations in scaling up the production and deployment of these soft, skin-surface glucose monitoring devices for widespread use in diabetes management?
Scaling up the production and deployment of soft, skin-surface glucose monitoring devices for widespread use in diabetes management may face several challenges and limitations. One significant challenge is ensuring the mass production of these devices while maintaining consistent quality and biocompatibility standards. This requires establishing efficient manufacturing processes and quality control measures to meet the demand for a large number of devices. Additionally, regulatory approval and compliance with medical device standards can pose obstacles to the widespread deployment of these devices, as ensuring safety and efficacy is crucial for their acceptance in clinical settings. Moreover, the cost-effectiveness of producing and distributing these devices on a large scale needs to be addressed to make them accessible to a broader population of diabetes patients. Furthermore, educating healthcare providers and patients about the benefits and usage of these devices is essential for their successful adoption and integration into diabetes management practices. Overcoming these challenges will be crucial in realizing the full potential of soft, skin-surface glucose monitoring devices for widespread use in diabetes care.
0
