Información - Pharmacology - # Negative Allosteric Modulation of Mu-Opioid Receptor to Enhance Naloxone's Overdose Reversal Efficacy
Discovery of a Negative Allosteric Modulator that Enhances the Efficacy of Naloxone in Reversing Opioid Overdose
Conceptos Básicos
A novel negative allosteric modulator compound that binds to the inactive conformation of the mu-opioid receptor can work cooperatively with naloxone to potently block opioid agonist signaling and effectively reverse opioid overdose effects in vivo.
Resumen
The content describes the discovery and characterization of a novel negative allosteric modulator (NAM) compound that targets the inactive conformation of the mu-opioid receptor (µOR). Key highlights:
The µOR is a well-established target for analgesia, but conventional opioid agonists cause serious adverse effects like addiction and respiratory depression, contributing to the opioid overdose epidemic.
The researchers screened a large DNA-encoded chemical library to identify a NAM compound that selectively binds to the inactive µOR conformation.
This NAM compound enhances the affinity and efficacy of naloxone, a key opioid overdose reversal molecule, in blocking opioid agonist signaling.
Using cryo-electron microscopy, the authors demonstrate that the NAM binds to the extracellular vestibule of µOR, directly interacting with the naloxone binding site and stabilizing an inactive receptor conformation.
In vivo, the NAM works cooperatively with low doses of naloxone to effectively inhibit various morphine-induced and fentanyl-induced behavioral effects while minimizing withdrawal symptoms.
The results provide structural insights into negative allosteric modulation of µOR and how it can be leveraged to enhance the therapeutic potential of naloxone in reversing opioid overdose.
A µ-opioid receptor modulator that works cooperatively with naloxone - Nature
Estadísticas
The µ-opioid receptor is a well-established target for analgesia.
Conventional opioid receptor agonists cause serious adverse effects, notably addiction and respiratory depression.
The current opioid overdose epidemic is driven by the highly potent synthetic opioid fentanyl.
Citas
"µOR negative allosteric modulators (NAMs) may serve as useful tools in preventing opioid overdose deaths, but promising chemical scaffolds remain elusive."
"The NAM works cooperatively with naloxone to potently block opioid agonist signalling."
"The NAM alters orthosteric ligand kinetics in therapeutically desirable ways and works cooperatively with low doses of naloxone to effectively inhibit various morphine-induced and fentanyl-induced behavioural effects in vivo while minimizing withdrawal behaviours."
Ideas clave extraídas de
by Evan... a las www.nature.com 07-03-2024
https://www.nature.com/articles/s41586-024-07587-7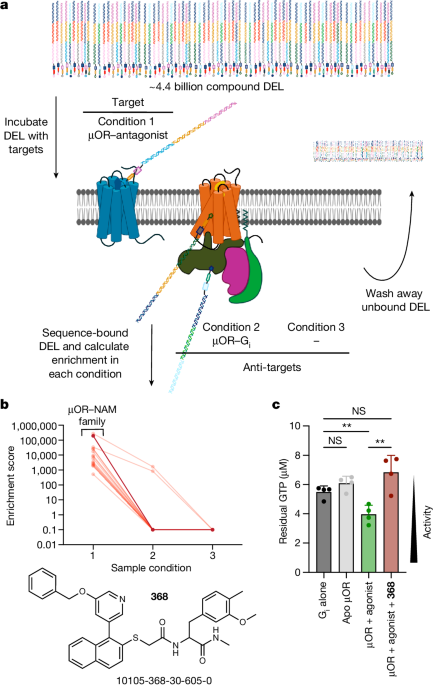
Consultas más profundas
How could this NAM compound be further optimized to enhance its therapeutic potential and safety profile?
To enhance the therapeutic potential and safety profile of this NAM compound, several optimization strategies could be considered. Firstly, structural modifications could be made to the compound to improve its pharmacokinetic properties, such as increasing its bioavailability or extending its half-life. This could involve altering the chemical structure to enhance its stability or metabolic profile in the body. Additionally, further medicinal chemistry efforts could focus on fine-tuning the binding affinity and selectivity of the NAM for the inactive µOR, ensuring optimal target engagement while minimizing off-target effects. Moreover, conducting preclinical studies to assess the compound's efficacy and safety in relevant animal models would be crucial in guiding further optimization efforts. By iteratively refining the compound through structure-activity relationship studies and in vivo testing, its therapeutic potential and safety profile could be significantly enhanced.
What are the potential limitations or drawbacks of using a NAM in combination with naloxone for opioid overdose reversal?
While the combination of a NAM with naloxone for opioid overdose reversal shows promise, there are potential limitations and drawbacks to consider. One limitation is the complexity of dosing regimens when using two different types of compounds, which may require careful titration to achieve the desired therapeutic effect. Additionally, the potential for drug-drug interactions between the NAM and naloxone could pose a risk of adverse effects or reduced efficacy. Furthermore, the long-term safety profile of using a NAM in combination with naloxone would need to be thoroughly evaluated, especially regarding the potential for tolerance or dependence with prolonged use. Another drawback is the need for further clinical trials to establish the efficacy and safety of this combination therapy in human subjects, which could be a time-consuming and resource-intensive process. Addressing these limitations and drawbacks through rigorous preclinical and clinical studies would be essential to ensure the successful translation of this approach to clinical practice.
What other receptor systems or disease contexts could this approach of leveraging negative allosteric modulation be applied to for therapeutic benefit?
The approach of leveraging negative allosteric modulation (NAM) could be applied to various other receptor systems and disease contexts to achieve therapeutic benefits. For example, in the field of neuroscience, targeting negative allosteric modulators of neurotransmitter receptors such as the dopamine receptor or serotonin receptor could offer novel treatment options for neuropsychiatric disorders like schizophrenia or depression. In the realm of cardiovascular medicine, modulating negative allosteric sites on adrenergic receptors could lead to the development of more effective treatments for heart failure or hypertension. Moreover, in the context of immune-related diseases, targeting negative allosteric modulators of cytokine receptors or immune checkpoint receptors could offer new avenues for immunotherapy in conditions like cancer or autoimmune disorders. By exploring the application of negative allosteric modulation across a diverse range of receptor systems and disease contexts, researchers can uncover innovative therapeutic strategies with the potential to address unmet medical needs.
0
