Comprehensive Multi-Omic Analysis of Temporal Adaptations to Endurance Exercise Training in Rats
Keskeiset käsitteet
Endurance exercise training induces widespread multi-omic changes across multiple tissues, revealing insights into the adaptive responses that promote whole-body health.
Tiivistelmä
This study presents a comprehensive multi-omic analysis of the temporal adaptations to endurance exercise training in male and female rats. The researchers profiled the transcriptome, proteome, metabolome, lipidome, phosphoproteome, acetylproteome, ubiquitylproteome, epigenome, and immunome in 19 tissues over 4 time points during an 8-week training program.
Key insights from the study:
- Thousands of shared and tissue-specific molecular alterations were identified, with sex differences found in multiple tissues.
- Temporal multi-omic and multi-tissue analyses revealed expansive biological insights into the adaptive responses to endurance training, including widespread regulation of immune, metabolic, stress response, and mitochondrial pathways.
- Many of the observed changes are relevant to human health, including non-alcoholic fatty liver disease, inflammatory bowel disease, cardiovascular health, and tissue injury and recovery.
- The data and analyses presented in this study provide valuable resources for understanding the multi-tissue molecular effects of endurance training, which are made publicly available.
Mukauta tiivistelmää
Kirjoita tekoälyn avulla
Luo viitteet
Käännä lähde
toiselle kielelle
Luo miellekartta
lähdeaineistosta
Siirry lähteeseen
www.nature.com
Temporal dynamics of the multi-omic response to endurance exercise training - Nature
Tilastot
The study profiled 9,466 assays across 19 tissues, 25 molecular platforms, and 4 training time points.
Lainaukset
"Regular exercise promotes whole-body health and prevents disease, but the underlying molecular mechanisms are incompletely understood."
"The resulting data compendium encompasses 9,466 assays across 19 tissues, 25 molecular platforms and 4 training time points."
"Many changes were relevant to human health, including non-alcoholic fatty liver disease, inflammatory bowel disease, cardiovascular health and tissue injury and recovery."
Tärkeimmät oivallukset
by David Amar,N... klo www.nature.com 05-01-2024
https://www.nature.com/articles/s41586-023-06877-w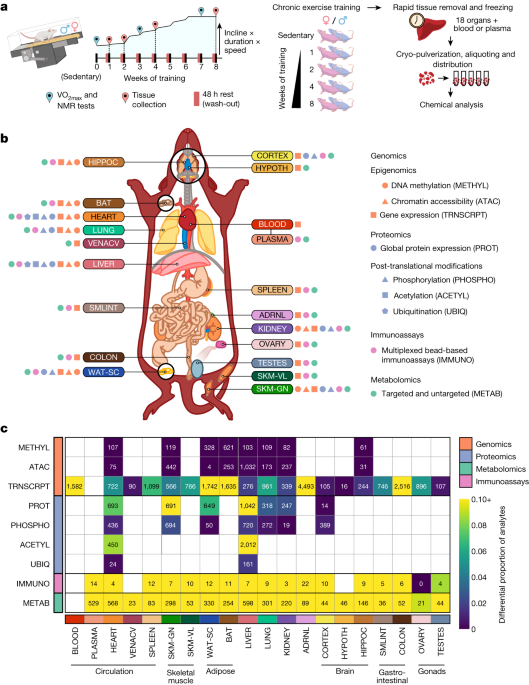
Syvällisempiä Kysymyksiä
How do the molecular adaptations to endurance training differ between healthy individuals and those with pre-existing conditions, such as metabolic or cardiovascular disorders?
In individuals with pre-existing metabolic or cardiovascular disorders, the molecular adaptations to endurance training may differ significantly from those in healthy individuals. For instance, individuals with metabolic disorders like non-alcoholic fatty liver disease may exhibit altered lipid metabolism pathways in response to exercise. Endurance training could lead to more pronounced improvements in insulin sensitivity and glucose metabolism in individuals with metabolic disorders compared to healthy individuals. Similarly, in individuals with cardiovascular disorders, exercise training may result in enhanced cardiac function, improved vascular health, and modulation of inflammatory pathways to a greater extent than in healthy individuals. The molecular adaptations in these populations may involve more robust changes in pathways related to oxidative stress, inflammation, and mitochondrial function to address the specific challenges posed by their pre-existing conditions.
What are the potential limitations of using a rodent model to study the multi-omic effects of exercise in humans, and how can these findings be validated and translated to human populations?
Using a rodent model to study the multi-omic effects of exercise in humans has several limitations. Firstly, rodents have different physiological and metabolic characteristics compared to humans, which may not fully capture the complexity of human responses to exercise. Additionally, the duration, intensity, and type of exercise that can be performed by rodents may not directly translate to human exercise regimens. Furthermore, the genetic variability between rodent strains and individual animals may limit the generalizability of findings to the diverse human population. To address these limitations, findings from rodent studies can be validated through translational research involving human subjects. This can include conducting controlled exercise interventions in human cohorts and comparing the molecular responses to those observed in rodent models. Additionally, leveraging human biobanks and multi-omic datasets can help validate the relevance of rodent findings to human populations.
What are the potential applications of the multi-omic data and insights from this study in the development of personalized exercise interventions or therapeutic targets for exercise-related health benefits?
The multi-omic data and insights from this study hold significant potential for the development of personalized exercise interventions and therapeutic targets for exercise-related health benefits. By identifying tissue-specific molecular alterations in response to endurance training, personalized exercise programs can be tailored to target specific pathways based on an individual's multi-omic profile. For example, individuals with a predisposition to inflammatory bowel disease may benefit from exercise interventions that modulate immune and inflammatory pathways identified in the study. Furthermore, the data can inform the development of novel therapeutic targets for conditions like cardiovascular disease or tissue injury, where exercise-induced molecular changes play a crucial role in promoting recovery and health. Integrating multi-omic data into clinical practice can enable healthcare providers to prescribe exercise regimens that are optimized for each individual's molecular profile, maximizing the health benefits derived from physical activity.
0
