näkemys - Computational Biology - # Alzheimer's Disease Pathology Visualized by Cryo-Electron Tomography
Detailed 3D Microscopy Reveals the Intricate Organization of Amyloid-β Plaques and Tau Tangles in Alzheimer's Disease
Keskeiset käsitteet
Cryo-electron tomography provides unprecedented 3D structural insights into the in situ organization of amyloid-β and tau filaments that form the characteristic plaques and tangles in Alzheimer's disease.
Tiivistelmä
The content discusses how the hallmark pathological features of Alzheimer's disease, namely amyloid-β plaques and tau tangles, are organized at the molecular level. Previous light microscopy studies have revealed the presence of these protein aggregates, but the precise arrangement of individual filaments within the plaques and tangles has remained unclear.
The authors used a technique called cryo-electron tomography to obtain high-resolution 3D images of brain tissue from Alzheimer's patients. This allowed them to visualize the intricate organization of amyloid-β and tau filaments that make up the plaques and tangles, respectively.
Key insights from the 3D microscopy data:
Amyloid-β filaments assemble into a highly organized, fibrillar structure within the plaques.
Tau filaments form twisted, intertwined strands that bundle together to create the characteristic tangles inside brain cells.
The spatial arrangement of these filamentous assemblies provides important clues about the pathogenic mechanisms underlying Alzheimer's disease.
Overall, this study leverages cutting-edge imaging technology to shed unprecedented light on the molecular architecture of the hallmark Alzheimer's pathologies, which could inform the development of targeted therapies.
Alzheimer’s plaques and tangles revealed by 3D microscopy
Tilastot
Alzheimer's disease was first described in 1907.
Amyloid-β peptides and tau proteins assemble into filaments that are thought to be harmful to the brain.
Lainaukset
"Amyloid-β filaments clump together in the space between brain cells and form plaques, whereas tau filaments form tangles and threads inside brain cells."
"These assemblies are visible under a light microscope and have been the defining features of Alzheimer's disease since it was first described in 1907."
Tärkeimmät oivallukset
by Sjors H. W. ... klo www.nature.com 07-10-2024
https://www.nature.com/articles/d41586-024-02119-9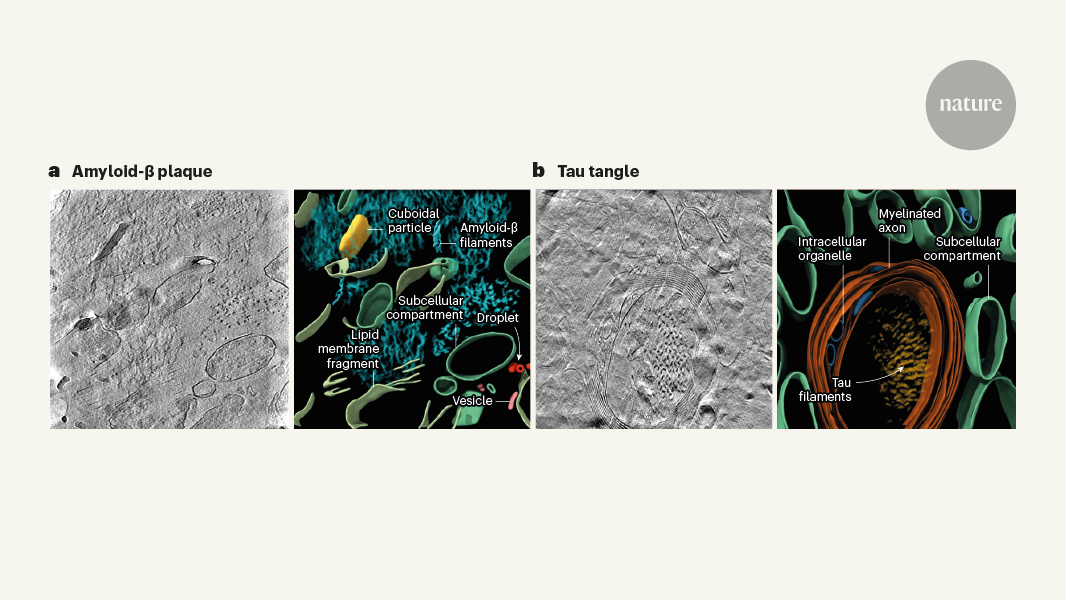
Syvällisempiä Kysymyksiä
How do the structural features of amyloid-β plaques and tau tangles revealed by cryo-electron tomography relate to their proposed mechanisms of toxicity and neurodegeneration?
The structural features of amyloid-β plaques and tau tangles play a crucial role in the proposed mechanisms of toxicity and neurodegeneration in Alzheimer's disease. Amyloid-β plaques are extracellular aggregates formed by the accumulation of amyloid-β peptides, which are believed to disrupt neuronal function and communication. The cryo-electron tomography images reveal the intricate arrangement of amyloid-β filaments within these plaques, providing insights into how these structures may interfere with synaptic activity and lead to neuronal damage. On the other hand, tau tangles are intracellular accumulations of hyperphosphorylated tau proteins, which destabilize microtubules and impair cellular transport mechanisms. The 3D visualization of tau filaments in tangles offers a detailed view of how these abnormal protein aggregates distort the internal architecture of neurons, contributing to their dysfunction and eventual degeneration. By elucidating the spatial organization of amyloid-β plaques and tau tangles at the molecular level, cryo-electron tomography helps connect the structural alterations to the proposed pathogenic pathways underlying Alzheimer's disease progression.
What are the potential limitations or caveats of the cryo-electron tomography approach used in this study, and how might they be addressed in future research?
While cryo-electron tomography provides unprecedented insights into the 3D organization of amyloid-β and tau filaments in Alzheimer's disease, there are certain limitations and caveats associated with this imaging technique. One challenge is the potential artifacts introduced during sample preparation, imaging, and data reconstruction, which could affect the accuracy of the structural details observed. Additionally, cryo-electron tomography requires specialized equipment and expertise, making it less accessible for widespread use in research settings. To address these limitations, future studies could focus on refining sample preparation protocols to minimize artifacts, optimizing imaging parameters to enhance resolution and contrast, and developing automated computational tools for data analysis and interpretation. Collaborative efforts among researchers with diverse expertise in cryo-electron microscopy and neurodegenerative diseases could also help overcome technical challenges and standardize protocols for more reliable and reproducible results.
Could the insights gained from visualizing Alzheimer's pathology at the molecular scale inspire the development of novel therapeutic strategies targeting the underlying protein aggregation processes?
The detailed visualization of Alzheimer's pathology at the molecular scale through cryo-electron tomography has the potential to inspire the development of novel therapeutic strategies aimed at targeting the underlying protein aggregation processes. By uncovering the precise structural arrangements of amyloid-β plaques and tau tangles, researchers can identify specific molecular interactions and conformational changes that drive the formation and propagation of these pathological aggregates. This knowledge could guide the design of small molecules, antibodies, or other therapeutic agents that selectively interfere with the aggregation pathways, prevent the accumulation of toxic protein species, or promote their clearance from the brain. Furthermore, understanding how these protein aggregates disrupt cellular functions and signaling pathways at the nanoscale level may reveal new targets for drug intervention to restore neuronal homeostasis and mitigate neurodegeneration in Alzheimer's disease. Ultimately, the insights gained from visualizing Alzheimer's pathology with molecular precision have the potential to revolutionize the development of targeted therapies that address the root causes of the disease.
0
