Structural Insights into the Stepwise Activation Mechanism of Metabotropic Glutamate Receptor Subtype 5
Concepts de base
The article presents a model for the sequential, multistep activation mechanism of metabotropic glutamate receptor subtype 5, revealing distinct receptor conformations upon agonist, allosteric modulator, and G protein binding.
Résumé
The content discusses the activation mechanism of metabotropic glutamate receptor subtype 5 (mGluR5), a G protein-coupled receptor (GPCR) that undergoes a large conformational change upon activation to transmit the ligand binding signal from the extracellular ligand-binding domain to the G protein-coupling 7-transmembrane domain.
The key highlights and insights are:
- Metabotropic glutamate receptors are obligate dimers with a large extracellular ligand-binding domain linked to a 7-transmembrane domain.
- The authors propose a model for a sequential, multistep activation mechanism of mGluR5, presenting a series of structures in lipid nanodiscs from inactive to fully active states, including agonist-bound intermediate states.
- Using bulk and single-molecule fluorescence imaging, the authors reveal distinct receptor conformations upon allosteric modulator and G protein binding.
- The study provides structural insights into the stepwise activation process of mGluR5, which is important for understanding the regulation and signaling of this class of GPCRs.
Personnaliser le résumé
Réécrire avec l'IA
Générer des citations
Traduire la source
Vers une autre langue
Générer une carte mentale
à partir du contenu source
Voir la source
www.nature.com
Stepwise activation of a metabotropic glutamate receptor - Nature
Stats
Metabotropic glutamate receptors are obligate dimers and possess a large extracellular ligand-binding domain linked to a 7-transmembrane domain.
Upon activation, these receptors undergo a large conformational change to transmit the ligand binding signal from the extracellular ligand-binding domain to the G protein-coupling 7-transmembrane domain.
Citations
"We present a series of structures in lipid nanodiscs, from inactive to fully active, including agonist-bound intermediate states."
"Further, using bulk and single-molecule fluorescence imaging, we reveal distinct receptor conformations upon allosteric modulator and G protein binding."
Idées clés tirées de
by Kaavya Krish... à www.nature.com 04-17-2024
https://www.nature.com/articles/s41586-024-07327-x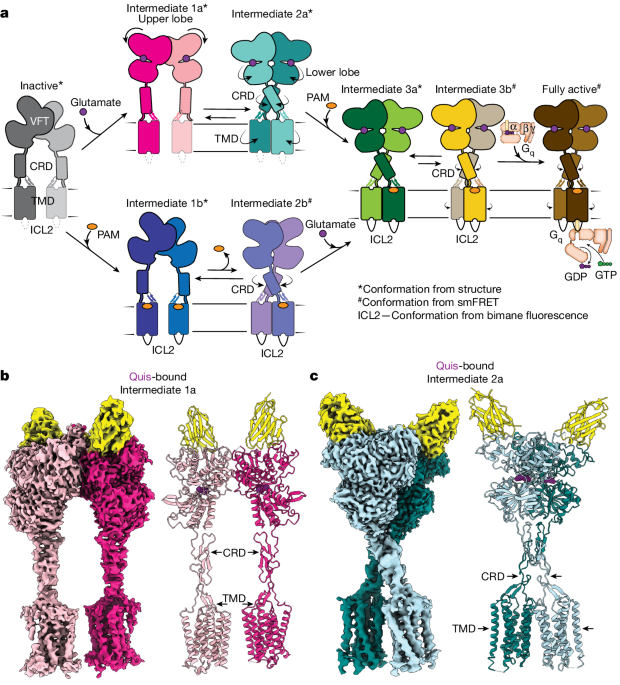
Questions plus approfondies
How do the distinct receptor conformations revealed in this study relate to the specific signaling pathways and functional outcomes mediated by mGluR5?
The distinct receptor conformations identified in this study play a crucial role in understanding the signaling pathways and functional outcomes mediated by mGluR5. As a member of the G protein-coupled receptor (GPCR) family, mGluR5 undergoes conformational changes upon activation, which are essential for transmitting the ligand binding signal and initiating downstream signaling cascades. By revealing these distinct receptor conformations, the study provides insights into the structural dynamics of mGluR5 during activation, shedding light on how different conformations correlate with specific signaling pathways and functional outcomes. This knowledge is vital for elucidating the molecular mechanisms underlying mGluR5 signaling and can potentially aid in the development of targeted therapies for conditions associated with mGluR5 dysfunction.
What are the potential implications of the proposed stepwise activation mechanism for the development of selective allosteric modulators or biased agonists targeting mGluR5?
The proposed stepwise activation mechanism of mGluR5 has significant implications for the development of selective allosteric modulators or biased agonists targeting this receptor. Understanding the sequential, multistep process of mGluR5 activation provides a roadmap for designing allosteric modulators that can modulate specific steps in the activation pathway. By targeting distinct conformations revealed in the study, researchers can develop allosteric modulators that selectively enhance or inhibit certain signaling pathways downstream of mGluR5 activation. Similarly, the insights into biased agonism gained from this activation mechanism can guide the design of biased agonists that preferentially activate specific signaling pathways while avoiding others. This precision in targeting mGluR5 activation pathways holds promise for the development of more effective and safer therapeutic agents with reduced off-target effects.
How might the insights from this study on the activation of mGluR5 be applied to understand the activation mechanisms of other classes of GPCRs?
The insights obtained from this study on the activation of mGluR5 can be extrapolated to enhance our understanding of the activation mechanisms of other classes of GPCRs. Since GPCRs share common structural and functional features, the stepwise activation mechanism proposed for mGluR5 may have broader implications for elucidating the activation processes of other GPCRs. By studying the conformational changes and signaling dynamics of mGluR5 in detail, researchers can draw parallels to other GPCRs and potentially uncover common activation principles that govern GPCR function. This comparative analysis can provide a framework for investigating the activation mechanisms of diverse GPCR subtypes and facilitate the development of novel therapeutic strategies targeting GPCR signaling pathways.
0
