Lysosomes Selectively Remove Damaged Sections of Mitochondrial Inner Membrane through Vesicle Formation
Alapfogalmak
Lysosomes selectively remove damaged sections of the mitochondrial inner membrane through a microautophagy-like process that forms vesicles derived from the inner mitochondrial membrane, thereby protecting the organelle from localized injury.
Kivonat
The article explores a novel pathway of intramitochondrial quality control, where lysosomes selectively remove damaged sections of the mitochondrial inner membrane (IMM) through a microautophagy-like process. Using super-resolution microscopy, the researchers found that cytosolic IMM vesicles, devoid of the outer mitochondrial membrane or mitochondrial matrix, are formed during the resting state. These vesicles derived from the IMM (VDIMs) are formed by IMM herniation through pores created by the voltage-dependent anion channel 1 in the outer mitochondrial membrane.
Live-cell imaging revealed that lysosomes in proximity to mitochondria engulf the herniating IMM, aided by the endosomal sorting complex required for transport machinery, leading to the formation of VDIMs while sparing the remainder of the organelle. This process was enhanced in mitochondria undergoing oxidative stress, suggesting the potential role of VDIMs in maintaining mitochondrial function.
Furthermore, the formation of VDIMs required calcium release by the reactive oxygen species-activated, lysosomal calcium channel, transient receptor potential mucolipin 1, demonstrating an interorganelle communication pathway for the maintenance of mitochondrial homeostasis. The researchers propose that IMM compartmentalization allows for the selective removal of damaged IMM sections via VDIMs, protecting the mitochondria from localized injury.
Lysosomes drive the piecemeal removal of mitochondrial inner membrane - Nature
Statisztikák
Mitochondrial membranes define distinct structural and functional compartments.
Cristae of the inner mitochondrial membrane (IMM) function as independent bioenergetic units that undergo rapid and transient remodelling.
Cytosolic IMM vesicles, devoid of outer mitochondrial membrane or mitochondrial matrix, are formed during resting state.
These vesicles derived from the IMM (VDIMs) are formed by IMM herniation through pores formed by voltage-dependent anion channel 1 in the outer mitochondrial membrane.
Formation of VDIMs was enhanced in mitochondria undergoing oxidative stress.
VDIM formation required calcium release by the reactive oxygen species-activated, lysosomal calcium channel, transient receptor potential mucolipin 1.
Idézetek
"Lysosomes in proximity to mitochondria engulfed the herniating IMM and, aided by the endosomal sorting complex required for transport machinery, led to the formation of VDIMs in a microautophagy-like process, sparing the remainder of the organelle."
"VDIM formation was enhanced in mitochondria undergoing oxidative stress, suggesting their potential role in maintenance of mitochondrial function."
"Our findings show a new pathway of intramitochondrial quality control."
Főbb Kivonatok
by Akriti Prash... : www.nature.com 08-21-2024
https://www.nature.com/articles/s41586-024-07835-w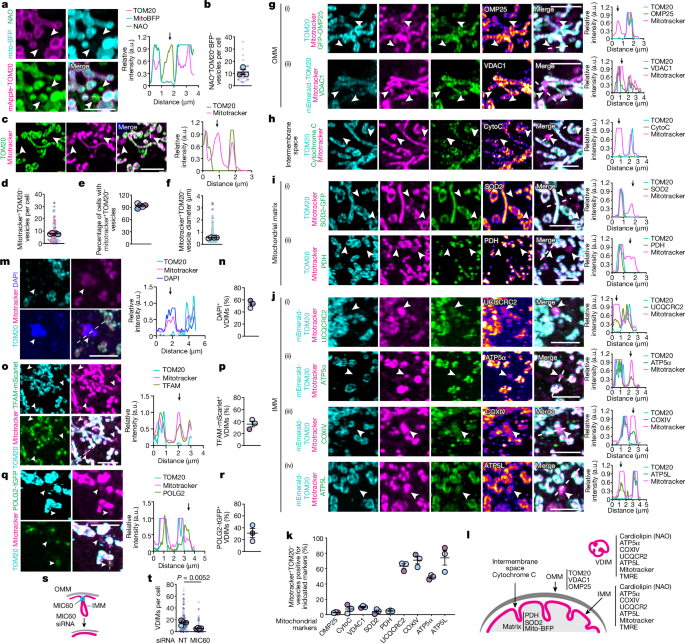
Mélyebb kérdések
How does the selective removal of damaged IMM sections via VDIMs contribute to the overall maintenance of mitochondrial function and cellular homeostasis?
The selective removal of damaged IMM sections via VDIMs plays a crucial role in maintaining mitochondrial function and cellular homeostasis by ensuring the integrity and efficiency of the organelle. By forming VDIMs, the cell can target and eliminate specific damaged regions of the IMM, preventing the spread of dysfunction to the entire mitochondrion. This process allows for the removal of compromised sections while sparing the healthy portions, thus preserving the overall functionality of the organelle. Additionally, by preventing the accumulation of damaged components, VDIM formation contributes to the overall quality control of mitochondria, ensuring that only functional units are retained within the cell.
What are the potential implications of disrupting the VDIM formation process, and how might this impact mitochondrial health and cellular function?
Disrupting the VDIM formation process could have significant implications for mitochondrial health and cellular function. Without the ability to selectively remove damaged IMM sections via VDIMs, dysfunctional regions may persist within the mitochondria, leading to a decline in overall organelle function. This could result in impaired energy production, compromised cellular metabolism, and increased susceptibility to oxidative stress. Furthermore, the accumulation of damaged components within the mitochondria could trigger further cellular damage and potentially contribute to the development of various diseases. Overall, disrupting the VDIM formation process could have detrimental effects on mitochondrial health and cellular function, impacting the overall well-being of the cell.
Given the interorganelle communication pathway involving lysosomes, mitochondria, and calcium signaling, what other cellular processes or pathways might be involved in the regulation of this mitochondrial quality control mechanism?
The interorganelle communication pathway involving lysosomes, mitochondria, and calcium signaling suggests a complex network of cellular processes involved in the regulation of mitochondrial quality control. Apart from the identified players, other cellular processes and pathways may also contribute to this mechanism. For instance, the ubiquitin-proteasome system could be involved in targeting specific proteins for degradation within the mitochondria. Additionally, autophagy pathways, such as macroautophagy and chaperone-mediated autophagy, may play a role in the clearance of damaged mitochondrial components. Mitochondrial dynamics, including fusion and fission events, could also influence the formation and removal of VDIMs. Moreover, signaling pathways related to cellular stress responses, such as the unfolded protein response and the heat shock response, may interact with the mitochondrial quality control mechanism to maintain cellular homeostasis. The intricate crosstalk between various cellular processes highlights the complexity of mitochondrial quality control and its regulation within the cell.
0
