Phage-Derived Enzyme Effectively Suppresses Graft-Versus-Host Disease by Targeting Pathogenic Enterococcus faecalis Biofilms
The article investigates the role of the gut microbiome in the pathogenesis of acute graft-versus-host disease (aGVHD) after allogeneic hematopoietic cell transplantation (allo-HCT). The researchers found that Enterococcus faecalis, a pathogenic bacterium, proliferates in the intestine by forming biofilms rather than acquiring drug resistance.
Through analysis of bacterial whole-genome sequencing data, the researchers identified an anti-E. faecalis enzyme derived from E. faecalis-specific bacteriophages. This enzyme was found to have lytic activity against E. faecalis biofilms both in vitro and in vivo. In aGVHD-induced gnotobiotic mice colonized with E. faecalis or patient fecal samples dominated by Enterococcus, treatment with the E. faecalis-specific enzyme significantly decreased the levels of intestinal cytolysin-positive E. faecalis and improved survival compared to controls.
The findings suggest that administration of this phage-derived antibacterial enzyme targeting biofilm-forming pathogenic E. faecalis could provide a novel approach to protect against aGVHD, which is difficult to treat with existing antibiotics.
Összefoglaló testreszabása
Átírás mesterséges intelligenciával
Hivatkozások generálása
Forrás fordítása
Egy másik nyelvre
Gondolattérkép létrehozása
a forrásanyagból
Forrás megtekintése
www.nature.com
An enterococcal phage-derived enzyme suppresses graft-versus-host disease - Nature
Főbb Kivonatok
by Kosuke Fujim... : www.nature.com 07-10-2024
https://www.nature.com/articles/s41586-024-07667-8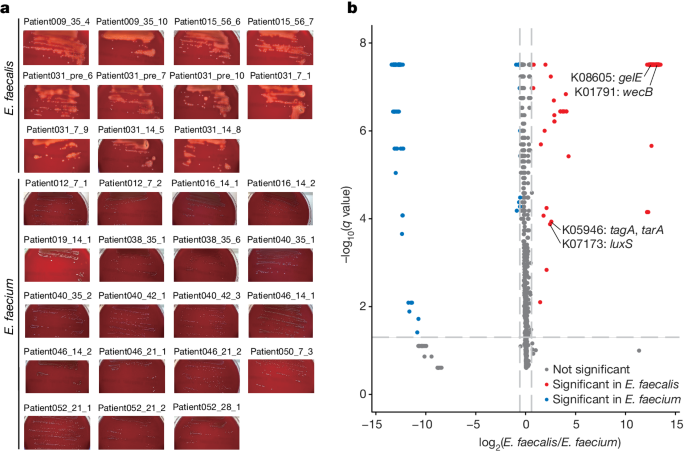
Mélyebb kérdések
