betekintés - Molecular Biology Microbiology - # Capsular Polysaccharide Secretion in Gram-negative Bacteria
Structural and Functional Insights into the Secretion Mechanism of Capsular Polysaccharides in Bacteria
Alapfogalmak
The ABC transporter KpsMT, associated with the KpsE and KpsD subunits, is responsible for the translocation of capsular polysaccharides (CPSs) across the inner bacterial membrane in Gram-negative bacteria.
Kivonat
The content provides structural and functional insights into the secretion mechanism of capsular polysaccharides (CPSs) in Gram-negative bacteria. Key highlights:
- CPSs are synthesized intracellularly on a lipid anchor and secreted across the cell envelope by the KpsMT ABC transporter associated with the KpsE and KpsD subunits.
- The study shows that KpsMT has broad substrate specificity and is sufficient for the translocation of CPSs across the inner bacterial membrane.
- Super-resolution fluorescence microscopy reveals the cell surface organization and localization of CPSs.
- Cryo-electron microscopy analyses of the KpsMT–KpsE complex in six different states provide insights into the conformational rearrangements of KpsMT during ATP hydrolysis and the recognition of a glycolipid inside a membrane-exposed electropositive canyon.
- In vivo CPS secretion assays highlight the functional importance of the canyon-lining basic residues.
- The findings suggest a molecular model of CPS secretion by ABC transporters.
Összefoglaló testreszabása
Átírás mesterséges intelligenciával
Hivatkozások generálása
Forrás fordítása
Egy másik nyelvre
Gondolattérkép létrehozása
a forrásanyagból
Forrás megtekintése
www.nature.com
Molecular insights into capsular polysaccharide secretion - Nature
Statisztikák
CPSs are synthesized intracellularly on a lipid anchor and secreted across the cell envelope by the KpsMT ABC transporter associated with the KpsE and KpsD subunits.
KpsMT has broad substrate specificity and is sufficient for the translocation of CPSs across the inner bacterial membrane.
Idézetek
"We show that KpsMT has broad substrate specificity and is sufficient for the translocation of CPSs across the inner bacterial membrane."
"Cryo-electron microscopy analyses of the KpsMT–KpsE complex in six different states reveal a KpsE-encaged ABC transporter, rigid-body conformational rearrangements of KpsMT during ATP hydrolysis and recognition of a glycolipid inside a membrane-exposed electropositive canyon."
Főbb Kivonatok
by Jeremi Kukle... : www.nature.com 04-03-2024
https://www.nature.com/articles/s41586-024-07248-9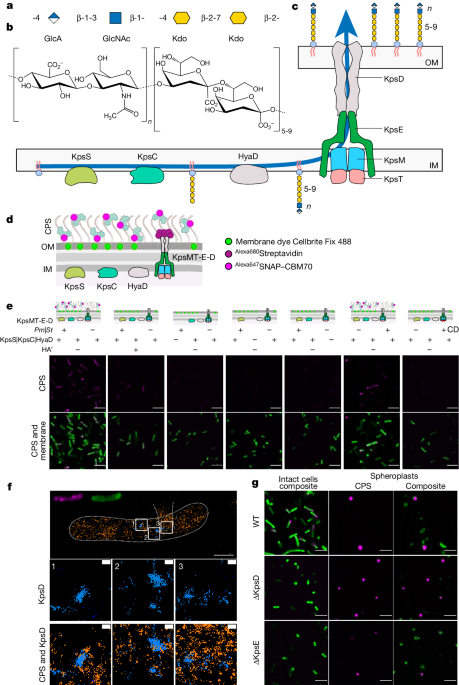
Mélyebb kérdések
How do the structural and functional insights into CPS secretion by the KpsMT ABC transporter contribute to the development of novel antimicrobial strategies targeting bacterial capsule formation?
The structural and functional insights into CPS secretion by the KpsMT ABC transporter provide a detailed understanding of the molecular mechanisms involved in bacterial capsule formation. By elucidating the crucial steps of CPS secretion, such as the broad substrate specificity of KpsMT and its role in translocating CPSs across the inner bacterial membrane, researchers can identify specific targets for novel antimicrobial strategies. For instance, the recognition of glycolipids inside a membrane-exposed electropositive canyon by the KpsMT-KpsE complex suggests potential sites for drug intervention to disrupt CPS secretion. Additionally, the in vivo CPS secretion assays highlighting the functional importance of canyon-lining basic residues could guide the development of inhibitors that specifically target these regions, thereby inhibiting bacterial capsule formation and potentially rendering bacteria more susceptible to immune responses or existing antibiotics.
What are the potential implications of the broad substrate specificity of KpsMT for the secretion of other bacterial surface structures beyond CPSs?
The broad substrate specificity of KpsMT opens up possibilities for its involvement in the secretion of other bacterial surface structures beyond CPSs. Understanding the versatility of KpsMT in translocating diverse substrates across the inner bacterial membrane suggests that this ABC transporter could play a role in the secretion of various components crucial for bacterial survival and virulence. For instance, KpsMT may be involved in the transport of lipopolysaccharides, proteins, or other cell envelope components that contribute to bacterial pathogenicity. Expanding the scope of KpsMT's substrate specificity could lead to the development of antimicrobial strategies that target multiple bacterial surface structures simultaneously, offering a more comprehensive approach to combating bacterial infections.
How might the understanding of the CPS secretion mechanism in Gram-negative bacteria inform our knowledge of polysaccharide transport processes in other biological systems, such as plant cell walls or the extracellular matrix of animal cells?
The understanding of the CPS secretion mechanism in Gram-negative bacteria can provide valuable insights into polysaccharide transport processes in other biological systems, such as plant cell walls or the extracellular matrix of animal cells. The structural and functional studies of the KpsMT-KpsE complex shed light on the intricate molecular interactions involved in translocating polysaccharides across membranes, which could be conserved in other systems. By studying the similarities and differences in the mechanisms of polysaccharide transport between bacteria and eukaryotic organisms, researchers can uncover fundamental principles that govern these processes across diverse biological systems. This cross-disciplinary approach may lead to the identification of common molecular targets or pathways that could be targeted for therapeutic interventions in various contexts, ranging from bacterial infections to plant diseases or disorders related to the extracellular matrix in animals.
0
