Adenosine Signaling Coordinates Astrocyte-Mediated Brain Metabolism and Function
Alapfogalmak
Adenosine signaling through astrocytic A2B receptors activates astrocyte glucose metabolism and lactate release, which is essential for supporting neuronal energy demands and maintaining synaptic function, sleep, and memory.
Kivonat
The article explores the mechanisms by which astrocytes, the glial cells in the brain, coordinate brain metabolism and function in response to neuronal activity. It demonstrates that the neuromodulator adenosine acts on astrocytic A2B receptors to activate the cAMP-PKA signaling pathway, leading to rapid stimulation of astrocyte glucose metabolism and lactate release. This astrocyte-mediated metabolic coupling is crucial for maintaining synaptic function, especially under conditions of high energy demand or reduced energy supply.
The key findings include:
- Neuronal activity-dependent metabolic activation of astrocytes is mediated by adenosine acting on astrocytic A2B receptors.
- Stimulation of A2B receptors recruits the cAMP-PKA signaling pathway in astrocytes, leading to increased glucose metabolism and lactate release.
- Conditional deletion of the A2B receptor gene in astrocytes impairs synaptic function, especially under high energy demand or reduced supply.
- Knockdown of A2B receptor expression in astrocytes leads to reprogramming of brain energy metabolism, prevents synaptic plasticity in the hippocampus, impairs recognition memory, and disrupts sleep.
- The adenosine A2B receptor acts as an astrocytic sensor of neuronal activity, and cAMP signaling in astrocytes tunes brain energy metabolism to support fundamental brain functions like sleep and memory.
Összefoglaló testreszabása
Átírás mesterséges intelligenciával
Hivatkozások generálása
Forrás fordítása
Egy másik nyelvre
Gondolattérkép létrehozása
a forrásanyagból
Forrás megtekintése
www.nature.com
Adenosine signalling to astrocytes coordinates brain metabolism and function - Nature
Statisztikák
Billions of nerve cells rely on a sufficient and uninterrupted nutrient and oxygen supply for brain computation.
Astrocytes govern brain glucose uptake and metabolism.
Experimental mouse models involving conditional deletion of the gene encoding A2B receptors in astrocytes showed that adenosine-mediated metabolic signaling is essential for maintaining synaptic function, especially under conditions of high energy demand or reduced energy supply.
Knockdown of A2B receptor expression in astrocytes led to a major reprogramming of brain energy metabolism, prevented synaptic plasticity in the hippocampus, severely impaired recognition memory, and disrupted sleep.
Idézetek
"Stimulation of A2B receptors recruits the canonical cyclic adenosine 3′,5′-monophosphate–protein kinase A signalling pathway, leading to rapid activation of astrocyte glucose metabolism and the release of lactate, which supplements the extracellular pool of readily available energy substrates."
"Experimental mouse models involving conditional deletion of the gene encoding A2B receptors in astrocytes showed that adenosine-mediated metabolic signalling is essential for maintaining synaptic function, especially under conditions of high energy demand or reduced energy supply."
"Knockdown of A2B receptor expression in astrocytes led to a major reprogramming of brain energy metabolism, prevented synaptic plasticity in the hippocampus, severely impaired recognition memory and disrupted sleep."
Főbb Kivonatok
by Shefeeq M. T... : www.nature.com 07-03-2024
https://www.nature.com/articles/s41586-024-07611-w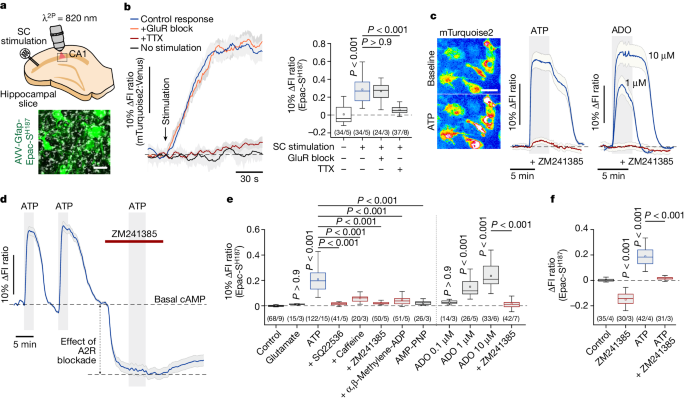
Mélyebb kérdések
How might the findings of this study be leveraged to develop therapeutic interventions for neurological disorders characterized by metabolic dysfunction, such as Alzheimer's disease or Parkinson's disease?
The findings of this study provide a crucial insight into the role of adenosine A2B receptor-mediated signaling in astrocytic metabolic activation, which is essential for supporting neuronal energy demands. Leveraging this knowledge, therapeutic interventions could target the adenosine A2B receptor pathway to modulate astrocyte glucose metabolism and lactate release, thereby potentially addressing metabolic dysfunction in neurological disorders like Alzheimer's disease or Parkinson's disease. By developing drugs that enhance A2B receptor activation or downstream cAMP-PKA signaling in astrocytes, it may be possible to restore proper brain energy metabolism and improve synaptic function in these conditions.
What other signaling pathways or mechanisms might be involved in the astrocyte-neuron metabolic coupling beyond the adenosine A2B receptor-cAMP-PKA axis?
While the adenosine A2B receptor-cAMP-PKA axis plays a significant role in astrocyte-neuron metabolic coupling, there are likely other signaling pathways and mechanisms involved in this intricate process. For instance, astrocytic glutamate uptake and metabolism, as well as interactions with other neurotransmitters and neuromodulators, could contribute to the coordination of brain energy metabolism. Additionally, signaling pathways related to calcium dynamics, such as the calcium-dependent activation of enzymes involved in glucose metabolism, may also play a role in astrocyte-neuron metabolic coupling. Further research is needed to elucidate the full spectrum of signaling pathways and mechanisms that contribute to the complex interplay between astrocytes and neurons in regulating brain metabolism.
Could the insights from this study on the role of astrocytic metabolism in supporting brain functions like memory and sleep be extended to understand the relationship between brain metabolism and cognitive aging or sleep disorders?
The insights gained from this study regarding the critical role of astrocytic metabolism in supporting brain functions like memory and sleep have the potential to be extended to understanding the relationship between brain metabolism and cognitive aging or sleep disorders. As cognitive aging and sleep disorders are associated with alterations in brain energy metabolism, including glucose utilization and lactate production, the mechanisms identified in this study, such as adenosine A2B receptor-mediated signaling in astrocytes, could offer valuable insights. By investigating how changes in astrocytic metabolic support impact cognitive aging processes or sleep regulation, researchers may uncover new therapeutic targets for addressing age-related cognitive decline or sleep disturbances through interventions that modulate astrocyte-neuron metabolic coupling.
0
