Glassy Polymers Transformed into Tough, Stretchable Gels through Ionic Liquid Swelling
The content describes a novel class of materials called "glassy gels" that combine the desirable properties of both glasses and gels. Typically, glassy polymers are stiff and strong but have limited extensibility. Swelling these polymers with solvents can make them soft and weak gels with enhanced extensibility.
The key innovation presented here is the use of ionic liquids to swell and toughen glassy polymers. The ionic liquids increase the free volume between polymer chains, enhancing extensibility, while also forming strong non-covalent crosslinks that render the material stiff, tough, and homogeneous (no phase separation).
Despite being over 54% liquid, these glassy gels exhibit impressive mechanical properties - high fracture strength (42 MPa), toughness (110 MJ/m^3), yield strength (73 MPa), and Young's modulus (1 GPa), comparable to thermoplastics like polyethylene. Crucially, they can be deformed up to 670% strain with full and rapid recovery on heating, unlike typical thermoplastics.
These transparent glassy gels are formed in a single-step polymerization process and also exhibit useful adhesive, self-healing, and shape-memory properties.
Összefoglaló testreszabása
Átírás mesterséges intelligenciával
Hivatkozások generálása
Forrás fordítása
Egy másik nyelvre
Gondolattérkép létrehozása
a forrásanyagból
Forrás megtekintése
www.nature.com
Glassy gels toughened by solvent - Nature
Főbb Kivonatok
by Meixiang Wan... : www.nature.com 06-19-2024
https://www.nature.com/articles/s41586-024-07564-0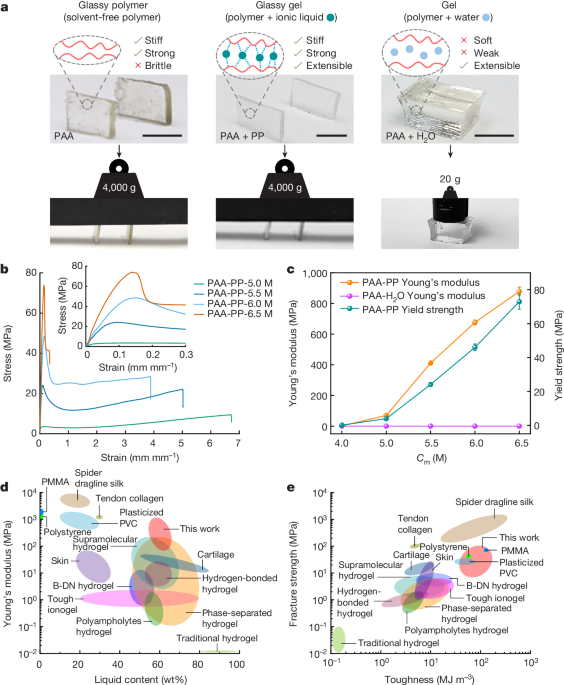
Mélyebb kérdések
