Roche's Breakthrough Blood Test for Measuring Lipoprotein(a) Levels to Assess Cardiovascular Risk
The content discusses a breakthrough blood test developed by Roche, in partnership with Amgen, that measures lipoprotein(a) [Lp(a)] levels. Lp(a) is a type of lipoprotein that is genetically inherited, and elevated levels have been associated with an increased risk of heart disease, stroke, and other blood vessel diseases.
The Tina-quant Lp(a) RxDx assay has received breakthrough device designation from the FDA, which is intended to expedite the development and review of devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. If approved, the test will be available on select Roche cobas platforms.
Elevated Lp(a) levels are prevalent in about 1 in 5 people worldwide, particularly among women and individuals of African descent. While low-density-lipoprotein (LDL) cholesterol is the primary risk factor for heart disease, Lp(a) has a higher atherogenic risk per particle compared to LDL cholesterol.
Currently, there are no approved pharmacologic therapies to lower Lp(a) levels in the United States, but several promising treatments, such as short interfering RNA (siRNA) agents, are in development to target the LPA gene and reduce Lp(a) synthesis.
Kustomisasi Ringkasan
Tulis Ulang dengan AI
Buat Sitasi
Terjemahkan Sumber
Ke Bahasa Lain
Buat Peta Pikiran
dari konten sumber
Kunjungi Sumber
www.medscape.com
Roche Blood Test for Lp(a) Designated Breakthrough Device
Wawasan Utama Disaring Dari
by Megan Brooks pada www.medscape.com 05-29-2024
https://www.medscape.com/viewarticle/roche-blood-test-lp-designated-breakthrough-device-2024a1000a3k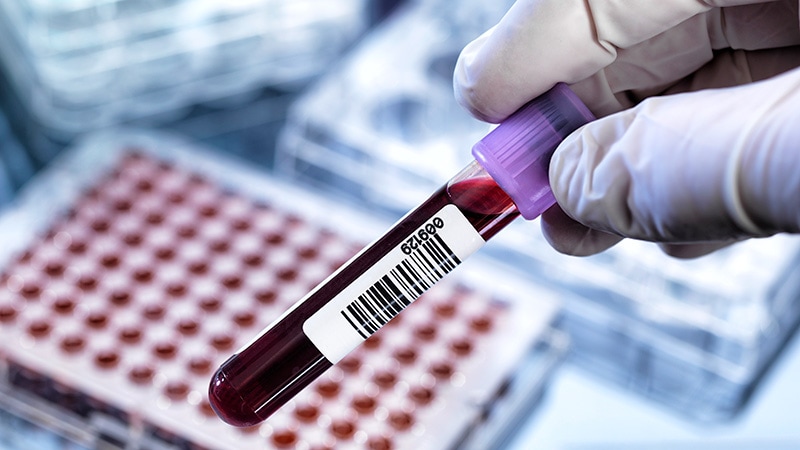
Pertanyaan yang Lebih Dalam
