Unleashing Macrophage Efferocytic Capacity Through Transcriptional Pause Release
Core Concepts
Macrophages utilize transcriptional pause/release to rapidly adapt their transcription and enhance efferocytosis efficiency.
Abstract
Macrophages employ transcriptional pause/release to enhance efferocytosis efficiency by controlling gene expression, particularly in corpse processing and phagosome acidification. This mechanism allows for the rapid alteration of transcriptional programs for effective processing of apoptotic corpses.
Rapid unleashing of macrophage efferocytic capacity via transcriptional pause release - Nature
Stats
RNA polymerase II initiates transcription for 20–60 nucleotides before being paused.
Blocking Pol II pause/release did not affect Fc receptor-mediated phagocytosis or bacterial phagocytosis.
Pol II pause/release controls the expression of select transcription factors and downstream target genes during efferocytosis.
Mechanistic studies on EGR3 revealed reprogramming of macrophage genes involved in cytoskeleton and corpse processing.
Pause/release plays a role in phagosome acidification during efferocytosis.
Quotes
"Macrophages use Pol II pause/release as a mechanism to rapidly alter their transcriptional programs."
"EGR3-related reprogramming of other macrophage genes involved in cytoskeleton and corpse processing."
"Pause/release is crucial for efficient processing of ingested apoptotic corpses."
Key Insights Distilled From
by Turan Tufan,... at www.nature.com 03-13-2024
https://www.nature.com/articles/s41586-024-07172-y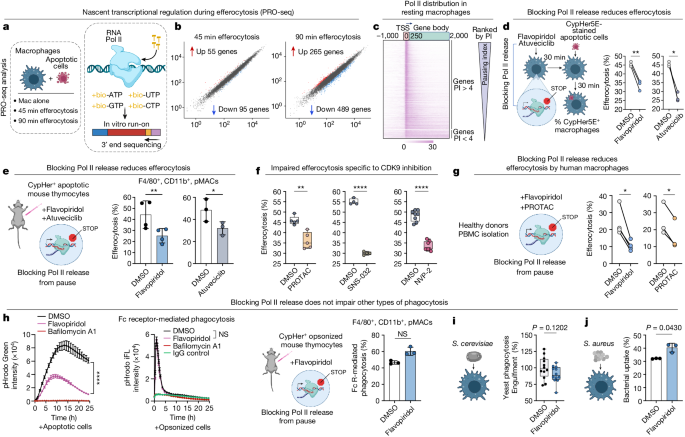
Deeper Inquiries
How does the utilization of Pol II pause/release impact the overall immune response?
The utilization of Pol II pause/release by macrophages plays a crucial role in shaping the overall immune response, particularly in the context of efferocytosis. By employing transcriptional pause/release mechanisms, macrophages can rapidly adapt their gene expression profiles to efficiently process apoptotic corpses and maintain tissue homeostasis. This rapid transcriptional response allows for continuous corpse uptake, which is essential for resolving inflammation and promoting tissue repair. Therefore, the ability of macrophages to utilize Pol II pause/release directly impacts their capacity to perform efferocytosis effectively, contributing to the regulation of immune responses during development, injury, or inflammatory conditions.
What potential implications could blocking Pol II pause/release have on macrophage function?
Blocking Pol II pause/release in macrophages could have significant implications on their function and ability to carry out efferocytosis efficiently. The interruption of this transcriptional mechanism may hinder the rapid adaptation of macrophages' gene expression profiles required for continuous corpse uptake. As demonstrated in studies with human and mouse macrophages, inhibiting Pol II pause/release resulted in impaired efferocytic capacity both in vitro and in vivo. Interestingly, while blocking this mechanism did not affect other phagocytic processes like Fc receptor-mediated phagocytosis or bacterial engulfment, it specifically impacted efferocytosis-related functions. Therefore, disrupting Pol II pause/release could lead to compromised clearance of apoptotic cells by macrophages and potentially contribute to unresolved inflammation or impaired tissue repair.
How can understanding macrophage transcriptional responses contribute to developing new therapeutic strategies?
Understanding how macrophage transcriptional responses are orchestrated during processes like efferocytosis provides valuable insights that can guide the development of novel therapeutic strategies for various diseases characterized by dysregulated immune responses or impaired tissue homeostasis. By deciphering the molecular mechanisms underlying transcriptional control in macrophages—such as the role of Pol II pause/release—researchers can identify key regulatory pathways that govern efficient corpse processing and resolution of inflammation. Targeting these specific pathways through pharmacological interventions or genetic manipulation holds promise for modulating macrophage function towards desired outcomes.
Moreover, insights into how transcription factors like EGR3 influence broader gene expression programs related to cytoskeleton dynamics and phagosomal acidification during efferocytosis offer potential targets for intervention. Therapeutic approaches aimed at manipulating these critical regulators identified through studying macropahge transcriptomic responses could lead to innovative treatments for conditions where defective clearance of apoptotic cells contributes to pathology.
0
