insight - Cell Biology - # Endoplasmic Reticulum-Plasma Membrane Contacts and Directional Cell Migration
Polarized Endoplasmic Reticulum-Plasma Membrane Contacts Guide Directional Cell Migration
Core Concepts
Polarized endoplasmic reticulum-plasma membrane contact sites, regulated by microtubule-driven ER structural changes, confine receptor signaling to the front and direct cell migration.
Abstract
The content describes how endoplasmic reticulum (ER)-plasma membrane (PM) contact sites are polarized in single and collectively migrating cells, and how this polarization directs cell migration.
Key highlights:
Directed cell migration is driven by front-back polarization of intracellular signaling, with a long-range inhibitory mechanism suppressing signaling at the back.
The authors found that ER-PM contact sites are polarized, with increased density at the back providing more access for the ER-resident PTP1B phosphatase to PM substrates.
This confines receptor signaling to the front and directs cell migration.
The polarization of ER-PM contacts is due to microtubule-regulated polarization of the ER, with more curved ER at the front and more flattened ER at the back.
The resulting ER curvature gradient leads to small, unstable ER-PM contacts only at the front, which flow backwards and grow to large, stable contacts at the back, forming the front-back ER-PM contact gradient.
This structural polarity mediated by ER-PM contact gradients polarizes cell signaling and directs cell migration.
Endoplasmic reticulum–plasma membrane contact gradients direct cell migration - Nature
Stats
Directed cell migration is driven by front-back polarization of intracellular signaling.
ER-PM contact sites are polarized in single and collectively migrating cells.
The increased density of ER-PM contacts at the back provides the ER-resident PTP1B phosphatase more access to PM substrates.
Quotes
"Equally important is a long-range inhibitory mechanism that suppresses signalling at the back to prevent the formation of multiple fronts."
"The polarization of ER–PM contacts is due to microtubule-regulated polarization of the ER, with more RTN4-rich curved ER at the front and more CLIMP63-rich flattened ER at the back."
Key Insights Distilled From
by Bo Gong,Jake... at www.nature.com 06-12-2024
https://www.nature.com/articles/s41586-024-07527-5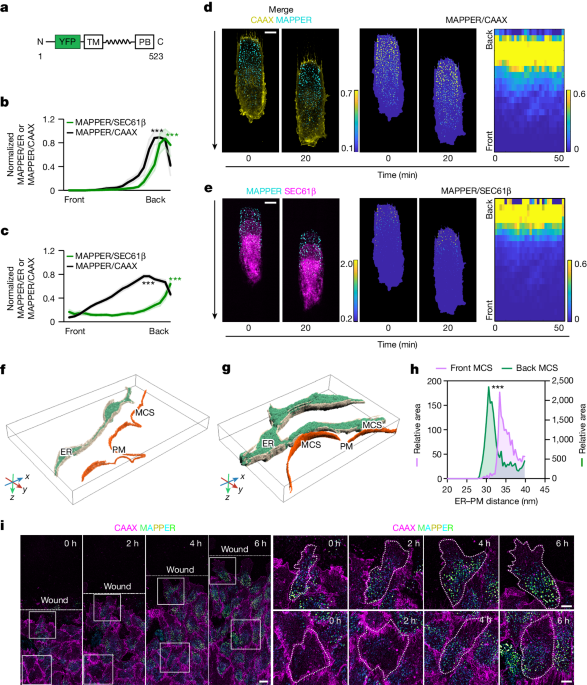
Deeper Inquiries
How might the findings on ER-PM contact gradients be leveraged to modulate cell migration in disease contexts, such as cancer metastasis or wound healing?
The discovery of polarized endoplasmic reticulum–plasma membrane (ER–PM) contact gradients opens up new possibilities for modulating cell migration in disease contexts. In cancer metastasis, where cells exhibit aberrant migratory behavior leading to the spread of cancer, targeting the polarization of ER–PM contacts could potentially be a therapeutic strategy. By manipulating the density and distribution of these contacts, it may be possible to restrict cell migration to specific directions, inhibiting the invasive nature of cancer cells. Similarly, in wound healing, where directed cell migration is crucial for tissue repair, understanding and controlling ER–PM contact gradients could enhance the speed and efficiency of wound closure. By promoting the formation of stable ER–PM contacts at the leading edge of migrating cells, wound healing processes could be accelerated.
What other cellular structures or organelle-membrane contact sites might exhibit similar polarized organization to direct cell behavior?
Apart from the ER–PM contact sites, other cellular structures and organelle-membrane contact sites may also exhibit polarized organization to direct cell behavior. One such example is the mitochondria–ER contact sites, known as mitochondria-associated membranes (MAMs). These sites play a crucial role in lipid transfer, calcium signaling, and apoptosis regulation. The polarized distribution of MAMs could influence cell metabolism, signaling pathways, and cell survival. Additionally, the Golgi apparatus, which is involved in protein processing and trafficking, may also show polarized organization at membrane contact sites to regulate cell secretion and migration. Understanding the dynamics of these organelle-membrane interactions could provide further insights into how cell behavior is orchestrated at the subcellular level.
Could the principles of ER-PM contact gradients be applied to engineer synthetic cell guidance systems or tissue engineering strategies?
The principles underlying ER-PM contact gradients could indeed be harnessed to engineer synthetic cell guidance systems and advance tissue engineering strategies. By mimicking the polarized organization of ER–PM contacts in artificial cell membranes or tissue scaffolds, researchers could design platforms that direct cell migration in desired directions. This could be particularly useful in regenerative medicine, where precise control over cell movement is essential for tissue repair and reconstruction. Synthetic cell guidance systems inspired by ER–PM contact gradients could be utilized to create more efficient wound healing therapies, organ-on-a-chip models, or bioengineered constructs for tissue regeneration. By leveraging these principles, innovative approaches to controlling cell behavior and tissue formation could be developed.
0
