Experimental Observation and Characterization of a Promethium Complex in Aqueous Solution
Core Concepts
Experimental observation and characterization of a stable chelation of the radioactive promethium (Pm) isotope in aqueous solution, providing fundamental insights into the lanthanide contraction phenomenon.
Abstract
The content describes an experimental study on the chemistry of promethium (Pm), the 61st element in the periodic table, which is highly radioactive and inaccessible. Despite the importance of Pm, it has been largely absent from experimental studies of lanthanides, hindering our understanding of the lanthanide contraction phenomenon.
The researchers demonstrate the successful chelation of the radioactive 147Pm isotope (half-life of 2.62 years) in aqueous solution using a newly synthesized organic diglycolamide ligand. They then use synchrotron X-ray absorption spectroscopy and quantum chemical calculations to establish the coordination structure and bond distance of the resulting homoleptic PmIII complex.
These fundamental insights allow the researchers to conduct a complete structural investigation of a full set of isostructural lanthanide complexes, capturing the lanthanide contraction in solution solely based on experimental observations. The results show an accelerated shortening of bonds at the beginning of the lanthanide series, which can be correlated to the separation trends exhibited by diglycolamides.
The characterization of the radioactive PmIII complex in an aqueous environment deepens the understanding of intra-lanthanide behavior and the chemistry and separation of the f-block elements.
Observation of a promethium complex in solution - Nature
Stats
The 147Pm radionuclide has a half-life of 2.62 years.
The bond distance of the promethium complex was established through the study.
Quotes
"Despite its importance, Pm has been conspicuously absent from the experimental studies of lanthanides, impeding our full comprehension of the so-called lanthanide contraction phenomenon: a fundamental aspect of the periodic table that is quoted in general chemistry textbooks."
"These fundamental insights allow a complete structural investigation of a full set of isostructural lanthanide complexes, ultimately capturing the lanthanide contraction in solution solely on the basis of experimental observations."
Key Insights Distilled From
by Darren M. Dr... at www.nature.com 05-22-2024
https://www.nature.com/articles/s41586-024-07267-6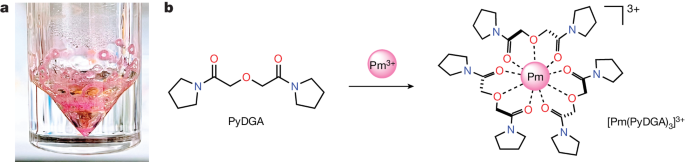
Deeper Inquiries
How can the insights gained from this study on promethium complexes be applied to improve the separation and purification of other f-block elements?
The insights obtained from studying promethium complexes can be instrumental in enhancing the separation and purification of other f-block elements. By understanding the coordination structure and bond distances of promethium complexes, researchers can apply similar chelation techniques and ligands to other lanthanides. This knowledge can aid in designing more efficient extraction processes for rare-earth elements, especially those with similar properties to promethium. Additionally, the accelerated shortening of bonds observed at the beginning of the lanthanide series can guide the development of selective extraction methods for specific lanthanides, contributing to improved separation and purification strategies for f-block elements.
What are the potential challenges and limitations in conducting experimental studies on highly radioactive elements like promethium, and how can they be addressed?
Experimental studies on highly radioactive elements like promethium present several challenges and limitations. One major obstacle is the handling of radioactive materials, which requires specialized facilities and stringent safety protocols to protect researchers from radiation exposure. Additionally, the short half-life of promethium (2.62 years) poses a challenge in conducting long-term experiments and obtaining stable samples for analysis. To address these challenges, researchers can utilize remote handling techniques, shielded environments, and robotic systems to minimize direct contact with radioactive materials. Moreover, collaboration with nuclear facilities and access to advanced spectroscopic and computational tools can facilitate the study of highly radioactive elements like promethium while ensuring the safety of researchers.
Given the importance of understanding the lanthanide contraction phenomenon, what other experimental techniques or theoretical approaches could be employed to further elucidate this fundamental aspect of the periodic table?
To further elucidate the lanthanide contraction phenomenon, researchers can employ a combination of experimental techniques and theoretical approaches. Experimentally, advanced spectroscopic methods such as X-ray crystallography, neutron scattering, and nuclear magnetic resonance (NMR) spectroscopy can provide detailed structural information on lanthanide complexes, allowing for precise determination of bond distances and coordination geometries. Additionally, time-resolved spectroscopy techniques can capture dynamic changes in lanthanide complexes, shedding light on their reactivity and coordination dynamics. Theoretical approaches, including density functional theory (DFT) calculations and molecular modeling, can complement experimental data by predicting the electronic structure, bonding interactions, and thermodynamic properties of lanthanide compounds. By integrating experimental and theoretical methods, researchers can gain a comprehensive understanding of the lanthanide contraction phenomenon and its implications in the chemistry of f-block elements.
0
