Guidelines for Ultrafast Li-ion Conduction in Batteries
Core Concepts
The author presents a novel approach to designing electrolytes for Li-ion batteries, focusing on small-sized solvents with low solvation energy to enhance ion conduction and enable high performance across various temperature ranges.
Abstract
The content discusses the challenges faced by Li-ion batteries in electric vehicles and aviation, emphasizing the need for high energy density, fast charging, and wide temperature operation. By utilizing small-sized solvents with low solvation energy, the author proposes a new electrolyte design concept that enhances Li+ transport through ligand channels while promoting the formation of an inorganic-rich interphase. This innovative approach is exemplified using fluoroacetonitrile (FAN) solvent, showcasing ultrahigh ionic conductivity at both room temperature and extreme cold conditions. The electrolyte's effectiveness enables high reversibility in pouch cells even at -65°C, highlighting its potential for extreme Li-ion battery applications.
Ligand-channel-enabled ultrafast Li-ion conduction - Nature
Stats
The electrolyte of 1.3 M lithium bis(fluorosulfonyl)imide (LiFSI) in FAN exhibits ultrahigh ionic conductivity of 40.3 mS cm−1 at 25 °C and 11.9 mS cm−1 even at −70 °C.
Achieves high reversibility (0.62 Ah) when charged and discharged even at -65°C.
Quotes
"The tiny solvent in the secondary solvation sheath pulls out the Li+ in the primary solvation sheath to form a fast ion-conduction ligand channel."
"The electrolyte with small-sized solvents enables LIBs to simultaneously achieve high energy density, fast charging, and a wide operating temperature range."
Key Insights Distilled From
by Di Lu,Ruhong... at www.nature.com 02-28-2024
https://www.nature.com/articles/s41586-024-07045-4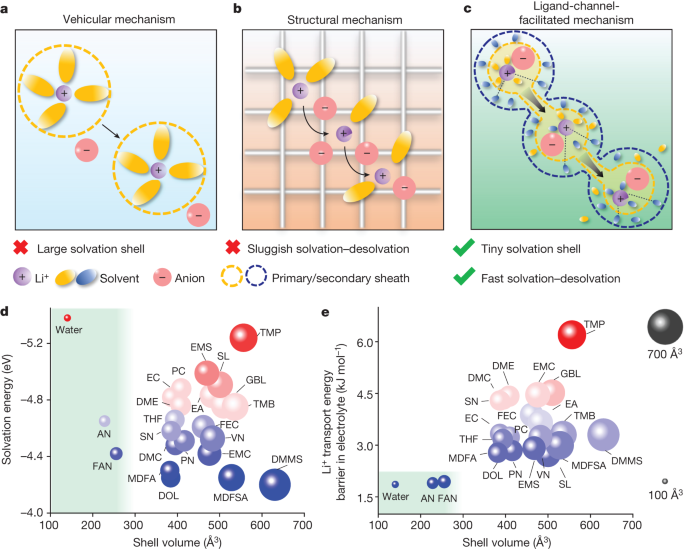
Deeper Inquiries
How can this innovative electrolyte design impact the future development of electric vehicle batteries
The innovative electrolyte design presented in the context has the potential to significantly impact the future development of electric vehicle batteries. By utilizing small-sized solvents with low solvation energy, this new approach enables high ionic conductivity, low solvation energy, and a wide operating temperature range simultaneously. This means that electric vehicle batteries can achieve higher energy density, faster charging capabilities, and improved performance even in extreme temperatures. The creation of ligand channels through the interaction between tiny solvents and Li+ ions enhances ion transport efficiency, leading to more efficient battery operation. Overall, this electrolyte design paves the way for next-generation electric vehicle batteries that are not only more powerful but also more reliable under various conditions.
What potential challenges or drawbacks could arise from implementing this new approach on a larger scale
While the novel electrolyte design offers promising benefits for electric vehicle batteries, there are potential challenges and drawbacks that could arise from implementing this approach on a larger scale. One concern is related to scalability and cost-effectiveness. The production of specialized electrolytes using specific small-sized solvents may require additional manufacturing processes or resources which could increase production costs. Additionally, there might be compatibility issues with existing battery components or materials when transitioning to these new electrolytes. Furthermore, ensuring long-term stability and safety of these advanced electrolytes under real-world conditions will be crucial before widespread adoption can occur.
How might advancements in metal-ion battery electrolytes contribute to sustainable energy storage solutions beyond current applications
Advancements in metal-ion battery electrolytes have the potential to contribute significantly to sustainable energy storage solutions beyond current applications. By improving key characteristics such as ionic conductivity, solvation energy, and operating temperature range as demonstrated in the context's research findings with fluoroacetonitrile (FAN) solvent-based electrolyte systems; it opens up possibilities for enhancing various types of metal-ion batteries used in renewable energy storage systems like grid-scale storage or residential solar installations.
Furthermore,
the ability to tailor electrode-electrolyte interactions at a molecular level allows for greater control over battery performance metrics such as cycle life,
rate capability,
and overall efficiency.
These advancements not only benefit traditional lithium-ion technologies but also pave
the way for emerging metal-ion chemistries like sodium-ion or potassium-ion batteries which hold promise for further expanding sustainable energy storage options on a global scale.
By pushing boundaries in electrochemical engineering through innovative
electrolyte designs,
we move closer towards achieving a cleaner
and more efficient future powered by renewable energies stored effectively within advanced metal-ion battery systems.
0
