FDA Approves Posluma for PET Imaging in Prostate Cancer
Core Concepts
FDA approves Posluma for PET imaging in prostate cancer.
Abstract
The US FDA has approved Posluma, a radiopharmaceutical for PET imaging in prostate cancer. Posluma binds to PSMA, aiding in disease extent evaluation. Available in the US from June 2023. Approval based on two single-arm trials showing high detection rates and minimal adverse events.
FDA Approval of Posluma
- FDA approves Posluma for PET imaging in prostate cancer.
- Posluma binds to PSMA, aiding in disease extent evaluation.
Availability and Manufacturer
- Posluma to be available in the US from June 2023.
- Manufactured and distributed by PETNET Solutions.
Comparison with Other Products
- Posluma compared to gallium-68 gozetotide for similar indications.
- Gozetotide also indicated for metastatic prostate cancer.
Approval Trials and Results
- Approval based on two single-arm trials from Blue Earth.
- LIGHTHOUSE trial showed low sensitivity but high specificity.
- SPOTLIGHT trial demonstrated high detection rates.
Adverse Events and Warnings
- Minimal adverse events reported in trials.
- Posluma PET contributes to long-term radiation exposure.
- Interpretation may vary among readers.
Customize Summary
Rewrite with AI
Generate Citations
Translate Source
To Another Language
Generate MindMap
from source content
Visit Source
www.medscape.com
Posluma Approved for PET Imaging in Prostate Cancer
Stats
Posluma binds prostate-specific membrane antigen (PSMA).
Posluma sensitivity for predicting positive nodes ranged from 23% to 30%.
Specificity of Posluma ranged from 93% to 97%.
Adverse events included diarrhea (0.7%), blood pressure increases (0.5%), and injection site pain (0.4%).
Quotes
"The study showed that Posluma PET provided clinically valuable information prior to surgery." - Brian Chapin, MD
Key Insights Distilled From
by M. Alexander... at www.medscape.com 05-31-2023
https://www.medscape.com/viewarticle/992577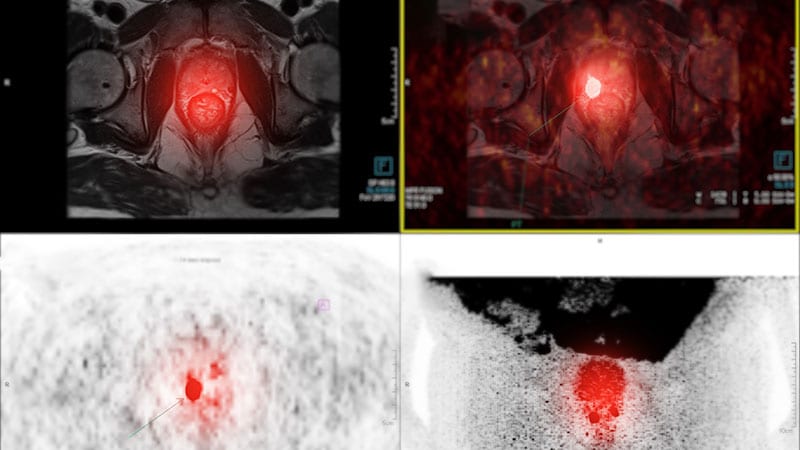
Deeper Inquiries
How does Posluma's approval impact the current standard of care for prostate cancer?
Posluma's approval for PET imaging in prostate cancer has significant implications for the current standard of care. By targeting prostate-specific membrane antigen (PSMA) overexpressed on prostate cancer cells, Posluma allows for more accurate imaging of the extent of disease. This can aid in the early detection of metastasis and recurrence, guiding treatment decisions. The approval of Posluma provides clinicians with a new tool to better assess and manage prostate cancer patients, potentially leading to more personalized and effective treatment strategies.
What are the potential implications of varying interpretations of Posluma PET results?
Varying interpretations of Posluma PET results could have several implications for patient care. Differences in interpretation among readers may lead to discrepancies in the diagnosis and staging of prostate cancer. This could result in variations in treatment decisions, potentially impacting patient outcomes. It is crucial for healthcare providers to establish standardized protocols for interpreting Posluma PET results to ensure consistency and accuracy in clinical practice. Additionally, ongoing education and training for readers can help minimize discrepancies and improve the reliability of Posluma PET imaging in prostate cancer management.
How can advancements in radiopharmaceuticals like Posluma shape the future of cancer imaging and treatment?
Advancements in radiopharmaceuticals like Posluma have the potential to revolutionize cancer imaging and treatment. By targeting specific biomarkers like PSMA, radiopharmaceuticals can provide precise and detailed information about tumor location, size, and spread. This can enable early detection, accurate staging, and monitoring of treatment response in cancer patients. Radiopharmaceuticals also offer the opportunity for theranostics, where imaging agents can be combined with therapeutic radionuclides for targeted cancer therapy. The development of novel radiopharmaceuticals like Posluma represents a promising avenue for personalized medicine, leading to more tailored and effective approaches to cancer diagnosis and treatment.
0
