Identification of PD-L1 as a Fungal-Binding Receptor through Phagosome Protein Profiling
Core Concepts
PD-L1 is identified as a receptor that directly binds to fungal proteins, enabling macrophages to cross-regulate cytokine production in response to fungal detection.
Abstract
This study developed a proximity labeling technique called PhagoPL to identify proteins that localize to phagosomes containing different microorganisms, such as yeast and bacteria. By comparing the protein composition of phagosomes with distinct microbes, the researchers unexpectedly found that programmed death-ligand 1 (PD-L1) specifically enriches in phagosomes containing yeast.
Further investigation revealed that PD-L1 directly binds to the fungal ribosomal protein Rpl20b upon processing in phagosomes. Using an auxin-induced depletion system, the authors demonstrated that detection of Rpl20b by macrophages can cross-regulate the production of cytokines, including interleukin-10 (IL-10), in response to the activation of other innate immune receptors.
This study establishes PhagoPL as a valuable approach to quantify the proteins enriched in phagosomes during host-microorganism interactions, leading to the identification of PD-L1 as a receptor that binds to fungi and modulates the immune response.
Profiling phagosome proteins identifies PD-L1 as a fungal-binding receptor - Nature
Stats
Phagocytosis is the process by which myeloid phagocytes bind to and internalize potentially dangerous microorganisms.
PD-L1 specifically enriches in phagosomes containing yeast, but not in phagosomes containing bacteria.
PD-L1 directly binds to the fungal ribosomal protein Rpl20b upon processing in phagosomes.
Detection of Rpl20b by macrophages can cross-regulate the production of cytokines, including interleukin-10 (IL-10), in response to the activation of other innate immune receptors.
Quotes
"By comparing the protein composition of phagosomes containing evolutionarily and biochemically distinct microorganisms, we unexpectedly identified programmed death-ligand 1 (PD-L1) as a protein that specifically enriches in phagosomes containing yeast."
"We found that PD-L1 directly binds to yeast upon processing in phagosomes."
"Using an auxin-inducible depletion system, we found that detection of Rpl20b by macrophages cross-regulates production of distinct cytokines including interleukin-10 (IL-10) induced by the activation of other innate immune receptors."
Key Insights Distilled From
by Kai Li,Avrad... at www.nature.com 06-05-2024
https://www.nature.com/articles/s41586-024-07499-6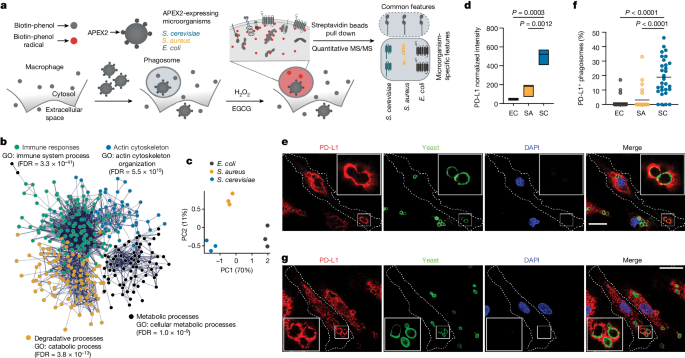
Deeper Inquiries
How might the binding of PD-L1 to fungal proteins like Rpl20b influence the overall immune response to fungal infections?
The binding of PD-L1 to fungal proteins like Rpl20b can have significant implications for the immune response to fungal infections. PD-L1, as a fungal-binding receptor, plays a crucial role in modulating the immune response by interacting with fungal proteins. This interaction can lead to the activation of downstream signaling pathways that regulate the production of cytokines, such as interleukin-10 (IL-10), which is known to have immunosuppressive effects. By binding to fungal proteins like Rpl20b, PD-L1 may influence the balance between pro-inflammatory and anti-inflammatory responses during fungal infections. This interaction could potentially dampen the immune response, allowing the fungus to evade immune detection and establish infection. Moreover, the binding of PD-L1 to fungal proteins may also impact antigen presentation and T cell activation, further shaping the overall immune response to fungal pathogens.
What other innate immune receptors or signaling pathways could be modulated by the detection of fungal proteins through PD-L1?
The detection of fungal proteins through PD-L1 could potentially modulate various innate immune receptors and signaling pathways involved in the immune response to fungal infections. One such receptor that could be influenced is Toll-like receptor 2 (TLR2), which is known to recognize fungal cell wall components and initiate pro-inflammatory responses. The interaction between PD-L1 and fungal proteins may cross-regulate TLR2 signaling, leading to altered cytokine production and immune responses. Additionally, the detection of fungal proteins through PD-L1 could impact other pattern recognition receptors (PRRs) such as Dectin-1, which recognizes β-glucans present in fungal cell walls. Modulation of Dectin-1 signaling by PD-L1-fungal protein interactions could affect phagocytosis, cytokine production, and the overall immune response to fungal pathogens.
Given the role of PD-L1 in regulating T cell responses, how might the fungal-binding function of PD-L1 impact adaptive immunity against fungal pathogens?
The fungal-binding function of PD-L1 can have significant implications for adaptive immunity against fungal pathogens, particularly in the context of T cell responses. PD-L1 is known to interact with its receptor PD-1 on T cells, leading to the inhibition of T cell activation and effector functions. In the presence of fungal proteins like Rpl20b, which bind to PD-L1, the interaction between PD-L1 and T cells may be altered. This could result in the suppression of T cell responses against fungal antigens, leading to impaired adaptive immunity. The fungal-binding function of PD-L1 may contribute to the establishment of fungal persistence by inhibiting T cell-mediated antifungal immune responses. Furthermore, the dysregulation of PD-L1-mediated T cell responses in the presence of fungal proteins could impact the balance between tolerance and immunity, ultimately influencing the outcome of fungal infections.
0
