Impact of Anti-TIGIT Antibody on PD-L1 Blockade Outcomes
Core Concepts
The author argues that the combination of tiragolumab and atezolizumab improves outcomes by activating macrophages and regulatory T cells, leading to a more favorable response in patients.
Abstract
In the phase 2 CITYSCAPE trial, combining tiragolumab with atezolizumab showed improved outcomes compared to atezolizumab alone. The presence of intratumoural macrophages and regulatory T cells was linked to better results with the combination treatment. Activation of macrophages was associated with clinical benefits in patients receiving the combined therapy. In mouse models, tiragolumab antibodies stimulated various immune cells, shifting CD8+ T cells from an exhausted state to a memory-like state. This study highlights how anti-TIGIT antibodies can reshape immunosuppressive tumor environments through FcγR engagement.
Anti-TIGIT antibody improves PD-L1 blockade through myeloid and Treg cells - Nature
Stats
Tiragolumab demonstrated improved outcomes in the phase 2 CITYSCAPE trial when combined with atezolizumab.
High baseline levels of intratumoural macrophages and regulatory T cells were associated with better outcomes in patients treated with atezolizumab plus tiragolumab.
Macrophage activation was linked to clinical benefits in patients who received the combination treatment.
Tiragolumab antibodies inflamed tumor-associated macrophages, monocytes, and dendritic cells through Fcγ receptors in mouse tumor models.
Quotes
Key Insights Distilled From
by Xiangnan Gua... at www.nature.com 02-28-2024
https://www.nature.com/articles/s41586-024-07121-9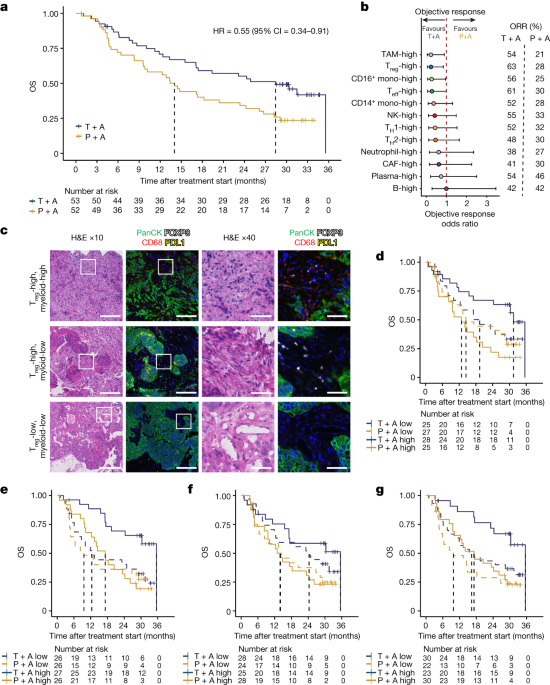
Deeper Inquiries
How might the findings of this study impact future developments in immunotherapy
The findings of this study could significantly impact future developments in immunotherapy by shedding light on the mechanism through which TIGIT checkpoint inhibitors, like tiragolumab, can remodel immunosuppressive tumor microenvironments. Understanding that targeting Fcγ receptors (FcγR) with anti-TIGIT antibodies can activate macrophages, monocytes, and dendritic cells to drive anti-tumor CD8+ T cells towards a more memory-like state opens up new avenues for enhancing the efficacy of cancer treatments. This insight may lead to the development of novel combination therapies that leverage both PD-L1 blockade and TIGIT inhibition to improve outcomes for patients with various types of cancer.
What potential drawbacks or limitations could arise from targeting FcγR engagement in anti-TIGIT antibody development
While targeting FcγR engagement in anti-TIGIT antibody development shows promise in remodeling immunosuppressive tumor microenvironments, there are potential drawbacks and limitations to consider. One concern is the risk of overstimulating immune responses, leading to autoimmune reactions or excessive inflammation. Additionally, variations in Fc receptor polymorphisms among individuals could affect the efficacy and safety profile of these antibodies. Careful consideration must be given to balancing immune activation with potential adverse effects when designing therapies that target FcγR engagement.
How can understanding the mechanisms behind checkpoint inhibitors lead to more effective cancer treatments
Understanding the mechanisms behind checkpoint inhibitors such as TIGIT can pave the way for more effective cancer treatments by enabling researchers and clinicians to tailor therapy approaches based on individual patient characteristics. By elucidating how these inhibitors interact with different immune cell populations within the tumor microenvironment, personalized treatment strategies can be developed to enhance anti-tumor immunity while minimizing off-target effects. This knowledge allows for a more precise selection of combination therapies that synergistically target multiple checkpoints or pathways involved in immune evasion by tumors, ultimately improving response rates and long-term outcomes for cancer patients.
0
