Advantages and Challenges of Flurpiridaz F-18 PET Tracer for Myocardial Perfusion Imaging
Core Concepts
Flurpiridaz F-18 PET tracer shows promise for improving myocardial perfusion imaging.
Abstract
The content discusses the emerging advantages and challenges of using the Flurpiridaz F-18 PET tracer for myocardial perfusion imaging. It highlights the benefits of PET MPI over SPECT MPI, the limitations of current tracers, the potential of Flurpiridaz F-18, FDA approval status, and the learning curve associated with this novel tracer.
Advantages of PET MPI
Better diagnostic performance and shorter acquisition times.
Consistent, high-quality images with low radiation exposure.
Ability to quantify myocardial blood flow and strong prognostic power.
Tracer Availability
Issues with current tracers limit widespread use in the clinic.
Rubidium and N-ammonia have limitations in availability and half-life.
15O-water is considered the gold standard but is challenging to use.
Flurpiridaz F-18
Novel PET MPI tracer with a longer half-life.
Potential to broaden the number of sites for perfusion PET studies.
Improved ability for quantification of perfusion and exercise PET.
FDA Approval
Flurpiridaz PET showed higher sensitivity and specificity than SPECT.
Expected FDA approval in 2024.
Learning Curve
Flurpiridaz comes with a learning curve due to its unique image characteristics.
Requires education and adaptation for accurate interpretation.
Novel PET Tracer for Perfusion Imaging: What's the Promise?
Stats
"Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and can be expensive for low-volume centers."
"N-ammonia, the other US FDA-approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron."
"For cardiac perfusion imaging and myocardial blood flow quantification, 15O-water is considered the gold standard."
"Flurpiridaz F-18, a novel PET MPI tracer, labeled with fluorine-18, has a longer half-life."
Quotes
"Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion."
"18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification."
Key Insights Distilled From
by Marilynn Lar... at www.medscape.com 10-19-2023
https://www.medscape.com/viewarticle/997548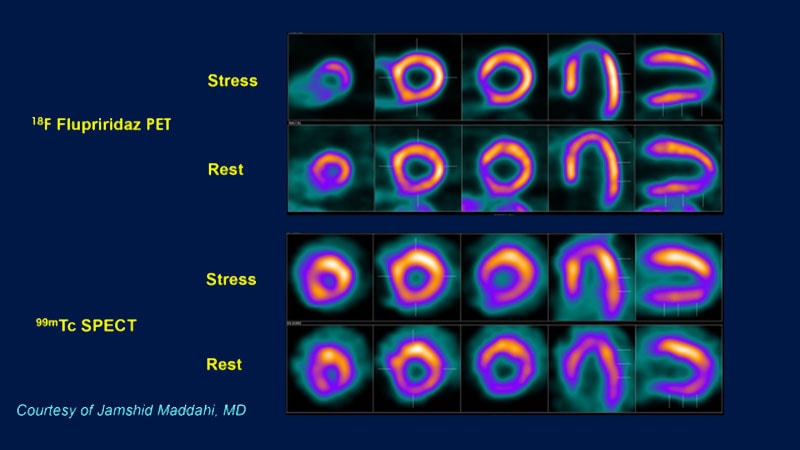
Deeper Inquiries
How might the FDA approval of Flurpiridaz impact the landscape of myocardial perfusion imaging?
The FDA approval of Flurpiridaz is expected to have a significant impact on the landscape of myocardial perfusion imaging. As a novel PET tracer labeled with fluorine-18, Flurpiridaz offers several advantages that could broaden the number of sites capable of performing perfusion PET studies. Its longer half-life compared to other tracers like rubidium and ammonia makes it more accessible and cost-effective for low-volume centers. Additionally, Flurpiridaz is designed to have a more linear relationship between flow and tracer uptake, which could enhance the ability to quantify perfusion accurately. The approval of Flurpiridaz is likely to increase access and availability of PET MPI studies, enable exercise stress testing, and optimize myocardial blood flow quantification, positioning cardiac PET as a leading modality for the functional evaluation of coronary artery disease.
What are the potential drawbacks or limitations of using Flurpiridaz F-18 as a PET tracer?
While Flurpiridaz F-18 shows promise as a novel PET tracer for myocardial perfusion imaging, there are potential drawbacks and limitations to consider. One limitation is the learning curve associated with interpreting images obtained with Flurpiridaz. The images produced by Flurpiridaz may appear different from those obtained with other tracers, requiring expertise and training to ensure accurate interpretation. Additionally, the performance of Flurpiridaz may not necessarily equate to that of other PET tracers used in prior studies, indicating the need for further research and comparison studies. Another limitation is the need for FDA approval, which requires rigorous phase-3 studies to demonstrate safety and diagnostic performance before widespread clinical use. Furthermore, the availability and cost of Flurpiridaz may still pose challenges for some centers, especially those without access to on-site cyclotrons or high-volume facilities.
How can the medical community ensure the quality and accuracy of interpreting images obtained with novel tracers like Flurpiridaz F-18?
Ensuring the quality and accuracy of interpreting images obtained with novel tracers like Flurpiridaz F-18 requires a multi-faceted approach within the medical community. First and foremost, ongoing education and training programs should be implemented to familiarize healthcare professionals with the unique characteristics and imaging patterns associated with Flurpiridaz. Professional societies, such as the American Society of Nuclear Cardiology, should play a key role in providing guidance and resources for interpreting images accurately. Collaboration between imaging experts, radiologists, and cardiologists is essential to share best practices and enhance the interpretation of PET MPI studies using Flurpiridaz. Additionally, continuous quality assurance measures, including regular audits and feedback mechanisms, can help identify and address any discrepancies or errors in image interpretation. By promoting a culture of continuous learning, collaboration, and quality improvement, the medical community can ensure that the introduction of novel tracers like Flurpiridaz leads to improved diagnostic accuracy and patient outcomes in myocardial perfusion imaging.
0
