Structural Insights into Integrator-Mediated RNA Polymerase II Termination Mechanism
Core Concepts
The Integrator complex uses a multi-step mechanism involving major structural rearrangements to terminate RNA polymerase II transcription by opening the DSIF DNA clamp and preventing Pol II rebinding.
Abstract
The content provides structural insights into how the Integrator complex terminates RNA polymerase II (Pol II) transcription. Key highlights:
-
Previous work has shown how Integrator binds to the paused Pol II elongation complex and cleaves the nascent RNA transcript, but the mechanism for removing Pol II from the DNA template was unknown.
-
The study presents three cryo-electron microscopy structures of the complete Integrator-PP2A complex in different functional states.
-
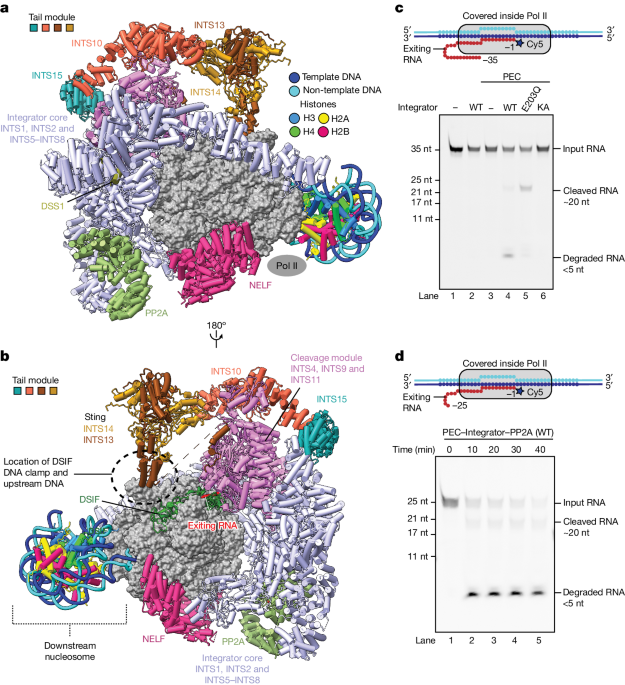
The pre-termination complex structure reveals a previously unresolved "scorpion-tail-shaped" INTS10-INTS13-INTS14-INTS15 module that may use its "sting" to open the DSIF DNA clamp and facilitate Pol II termination.
-
The post-termination complex structure shows that the unresolved INTS3 subunit and associated SOSS factors prevent Pol II from rebinding to Integrator after termination.
-
The structure of the free Integrator-PP2A complex in an inactive closed conformation reveals that INTS6 blocks the PP2A phosphatase active site.
-
These structural insights lead to a model of a three-step Integrator-mediated Pol II termination mechanism involving major conformational changes.
Translate Source
To Another Language
Generate MindMap
from source content
Visit Source
www.nature.com
Structural basis of Integrator-dependent RNA polymerase II termination - Nature
Stats
The Integrator complex can terminate RNA polymerase II (Pol II) in the promoter-proximal region of genes.
The structure of the pre-termination complex reveals a previously unresolved, scorpion-tail-shaped INTS10–INTS13–INTS14–INTS15 module.
The structure of the post-termination complex shows that the previously unresolved subunit INTS3 and associated sensor of single-stranded DNA complex (SOSS) factors prevent Pol II rebinding to Integrator after termination.
The structure of the free Integrator–PP2A complex in an inactive closed conformation reveals that INTS6 blocks the PP2A phosphatase active site.
Quotes
"The structure of the pre-termination complex reveals a previously unresolved, scorpion-tail-shaped INTS10–INTS13–INTS14–INTS15 module that may use its 'sting' to open the DSIF DNA clamp and facilitate termination."
"The structure of the post-termination complex shows that the previously unresolved subunit INTS3 and associated sensor of single-stranded DNA complex (SOSS) factors prevent Pol II rebinding to Integrator after termination."
"The structure of the free Integrator–PP2A complex in an inactive closed conformation reveals that INTS6 blocks the PP2A phosphatase active site."
Deeper Inquiries
How do the structural rearrangements of the Integrator complex during the three-step termination mechanism relate to its regulation and coordination with other cellular processes?
The structural rearrangements of the Integrator complex play a crucial role in its regulation and coordination with other cellular processes. During the three-step termination mechanism, the Integrator complex undergoes major rearrangements that are essential for its function. The scorpion-tail-shaped INTS10–INTS13–INTS14–INTS15 module in the pre-termination complex acts as a key player in opening the DSIF DNA clamp, facilitating termination. This structural change allows Integrator to interact with the paused elongation complex and cleave the nascent RNA transcript. Furthermore, the post-termination complex structure reveals how INTS3 and SOSS factors prevent Pol II rebinding, ensuring proper termination of transcription. These structural rearrangements not only enable Integrator to carry out its termination function but also regulate its interaction with other cellular components involved in transcriptional processes, highlighting the intricate coordination required for efficient gene expression regulation.
What are the potential implications of the Integrator-mediated Pol II termination mechanism for gene expression control and dysregulation in disease states?
The Integrator-mediated Pol II termination mechanism has significant implications for gene expression control and dysregulation in disease states. Dysregulation of transcription termination can lead to aberrant gene expression patterns, contributing to various diseases. The precise regulation of Pol II termination by the Integrator complex ensures the accurate expression of genes by controlling when and where transcription stops. Any disruptions in this process can result in the misregulation of gene expression, potentially leading to disease states. Understanding the structural basis of Integrator-dependent Pol II termination provides insights into how dysregulation of this mechanism can impact gene expression control, offering potential targets for therapeutic interventions aimed at restoring proper transcriptional regulation in disease conditions.
Could the insights into the Integrator complex structure and function inspire the development of new therapeutic strategies targeting transcriptional regulation?
The insights gained from studying the Integrator complex structure and function hold great promise for inspiring the development of new therapeutic strategies targeting transcriptional regulation. By elucidating the molecular mechanisms underlying Integrator-mediated Pol II termination, researchers can identify key components and interactions that can be targeted for therapeutic intervention. For instance, the discovery of the scorpion-tail-shaped module and its role in termination suggests that molecules disrupting this interaction could modulate transcriptional activity. Additionally, understanding how INTS6 blocks the PP2A phosphatase active site in the inactive conformation opens up possibilities for designing small molecules that can inhibit this interaction, potentially altering transcriptional outcomes. Overall, the detailed structural insights into the Integrator complex provide a foundation for the development of novel therapeutic approaches aimed at modulating transcriptional processes for the treatment of various diseases.