insight - Molecular Biology Genetics - # Mitochondrial DNA Polymerase Gamma Variant and Antiviral Tolerance
Ancestral DNA Polymerase Gamma Variant Modulates Antiviral Tolerance and Disease Manifestation in Mitochondrial Ataxia Syndrome
Core Concepts
The ancestral p.W748S variant in the mitochondrial DNA replicase POLG1 compromises antiviral defense mechanisms, leading to variable disease manifestations in mitochondrial recessive ataxia syndrome (MIRAS) patients.
Abstract
The content discusses the role of the mitochondrial DNA replicase POLG1 in antiviral defense mechanisms and how a common European founder mutation, the p.W748S variant, can impact disease manifestation in mitochondrial recessive ataxia syndrome (MIRAS).
Key highlights:
Mitochondria play a critical role in antiviral tolerance through the release of mitochondrial RNA and DNA (mtDNA and mtRNA) fragments, which activate virus sensors and the type-I interferon (IFN-I) response.
MIRAS, caused by the POLG1 p.W748S variant, shows variable ages of onset and symptoms, suggesting the presence of unknown modifying factors.
The study found that POLG1 has a role in antiviral defense against double-stranded DNA and positive-strand RNA viruses, and the p.W748S variant dampens innate immune responses.
The p.W748S variant compromises mtDNA replisome stability, leading to mtDNA depletion, which is further aggravated by virus infection.
Low mtDNA and mtRNA release into the cytoplasm and a slow IFN response in MIRAS offer viruses an early replicative advantage, leading to an augmented pro-inflammatory response, neuronal loss, and liver inflammation and necrosis.
A population databank analysis showed an enrichment of immunodeficient traits in carriers of the POLG1 p.W748S mutation.
The findings suggest that POLG1 defects compromise antiviral tolerance, triggering epilepsy and liver disease, with important implications for the mitochondrial disease spectrum.
Ancestral allele of DNA polymerase gamma modifies antiviral tolerance - Nature
Stats
Patients homozygous for the MIRAS variant p.W748S show exceptionally variable ages of onset and symptoms.
A population databank of around 300,000 Finnish individuals demonstrates enrichment of immunodeficient traits in carriers of the POLG1 p.W748S mutation.
Quotes
"Our patient and knock-in mouse data show that p.W748S compromises mtDNA replisome stability, causing mtDNA depletion, aggravated by virus infection."
"Low mtDNA and mtRNA release into the cytoplasm and a slow IFN response in MIRAS offer viruses an early replicative advantage, leading to an augmented pro-inflammatory response, a subacute loss of GABAergic neurons and liver inflammation and necrosis."
Key Insights Distilled From
by Yilin Kang,J... at www.nature.com 04-03-2024
https://www.nature.com/articles/s41586-024-07260-z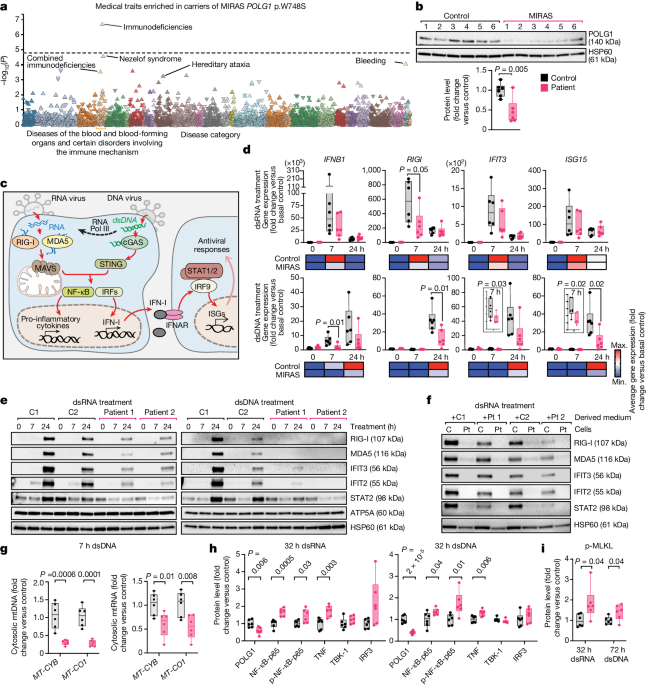
Deeper Inquiries
How do the mitochondrial defects associated with the POLG1 p.W748S variant impact other cellular processes beyond antiviral defense?
The mitochondrial defects linked to the POLG1 p.W748S variant have broader implications beyond antiviral defense mechanisms. One significant impact is on cellular energy production. Mitochondria play a crucial role in generating adenosine triphosphate (ATP) through oxidative phosphorylation, and any disruption in mitochondrial function, such as the depletion of mtDNA caused by the p.W748S variant, can lead to decreased ATP production. This reduction in ATP levels can affect various cellular processes that require energy, including metabolism, signaling pathways, and overall cellular homeostasis. Additionally, impaired mitochondrial function can result in the generation of reactive oxygen species (ROS), leading to oxidative stress and potential damage to cellular components like proteins, lipids, and DNA. Furthermore, mitochondria are involved in calcium homeostasis, apoptosis regulation, and the production of metabolites essential for cellular functions. Therefore, the mitochondrial defects associated with the POLG1 p.W748S variant can have far-reaching consequences on multiple cellular processes beyond antiviral defense.
What are the potential therapeutic strategies to enhance antiviral tolerance in individuals carrying the POLG1 p.W748S mutation?
Several therapeutic strategies can be considered to enhance antiviral tolerance in individuals carrying the POLG1 p.W748S mutation. One approach could involve targeting the mitochondrial dysfunction caused by the variant. This could include the use of mitochondrial-targeted antioxidants to mitigate oxidative stress and preserve mitochondrial function. Another strategy could focus on boosting the innate immune response by modulating the expression or activity of key antiviral proteins or cytokines involved in the immune response to viral infections. Additionally, enhancing the stability of the mtDNA replisome and promoting mtDNA replication could help maintain sufficient levels of mtDNA, thereby improving antiviral defense mechanisms. Furthermore, personalized antiviral therapies tailored to the specific viral infections encountered by individuals with the POLG1 p.W748S mutation could be developed to provide targeted and effective treatment. Overall, a combination of approaches targeting mitochondrial function, innate immune responses, and specific antiviral mechanisms may offer promising therapeutic strategies to enhance antiviral tolerance in individuals carrying the POLG1 p.W748S mutation.
What other genetic or environmental factors may interact with the POLG1 p.W748S variant to modulate the clinical presentation of mitochondrial diseases?
The clinical presentation of mitochondrial diseases associated with the POLG1 p.W748S variant can be influenced by various genetic and environmental factors. One genetic factor that may interact with the p.W748S variant is the presence of other mutations in mitochondrial DNA or nuclear genes encoding mitochondrial proteins. These additional genetic variants can exacerbate mitochondrial dysfunction, leading to more severe disease manifestations. Moreover, polymorphisms in genes involved in immune responses, inflammation, or cellular stress pathways could modulate the clinical presentation of mitochondrial diseases in individuals with the p.W748S variant. Environmental factors such as exposure to toxins, infections, or stressors that impact mitochondrial function or immune responses can also interact with the p.W748S variant to influence disease outcomes. Furthermore, lifestyle factors like diet, exercise, and medication use can affect mitochondrial health and potentially modify the clinical phenotype of mitochondrial diseases. Understanding the complex interplay between genetic and environmental factors is crucial for elucidating the full spectrum of clinical presentations associated with the POLG1 p.W748S variant in mitochondrial diseases.
0
